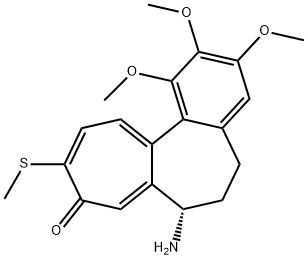
thiocolchicine synthesis
- Product Name:thiocolchicine
- CAS Number:2730-71-4
- Molecular formula:C22H25NO5S
- Molecular Weight:415.5

64-86-8
645 suppliers
$11.00/100mg
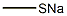
5188-07-8
263 suppliers
$13.00/5g

2730-71-4
75 suppliers
$30.00/5mg
Yield: 95%
Reaction Conditions:
in water for 1 h;Heating;
Steps:
General Procedure for the Synthesis of 10-alkylthio ColchicineAnalogues 2-6
General procedure: Synthesis of 10-thioalkylcolchicine derivatives is thesubject of patent registration [20]: Colchicine (0.5g, 0,00125Mol) and sodium alkylthiolates (CH3NaS, C2H5NaS, C3H7NaS, (CH3)2CHNaS and C4H9NaS) were commercialproducts of Fluka. To a water solution of colchicine, sodium methane thiolate, sodium ethane thiolate, sodium 1-propanethiolate, sodium 2-propanethiolate and sodium 1-buthanethiolate (0,01 Mol for 2, 0,0125 Mol for 6) were added, respectively. The reaction mixtures were heated (1h for 2, 48h for 6) and stirred. The product was dissolved in water (10-20mL) and 2% water solution of acetic acid (5-7mL) was added and extracted with chloroform (50mL 6-8 times), to yield 99% of 2, 96% of 3, 97% of 4, 94% of 5 and99% of 6, respectively. After purification using column chromatography on Florisil 60-100 mesh (Fluka) by solvent mixture: acetone: toluene: ethyl acetate: chloroform at the ratio 1:1:1:1 the alkylthio derivatives were obtained with very good yield from the range 72 - 95%. The yellow crystalline product of 10-ethylthiocolchicine was obtained and recrystallized from ethyl acetate to obtain single crystals suitable for X-ray analysis.
References:
Kurek, Joanna;Boczon, Wladyslaw;Myszkowski, Krzysztof;Murias, Marek;Borowiak, Teresa;Wolska, Irena [Letters in drug design and discovery,2014,vol. 11,# 3,p. 279 - 289]

64-86-8
645 suppliers
$11.00/100mg

2730-71-4
75 suppliers
$30.00/5mg
74-93-1
121 suppliers
$454.02/5MG

64-86-8
645 suppliers
$11.00/100mg

2730-71-4
75 suppliers
$30.00/5mg
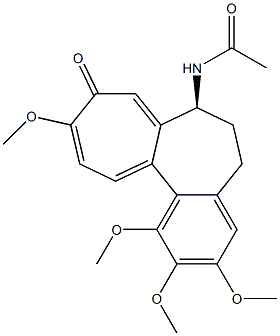
209810-38-8
11 suppliers
inquiry

2730-71-4
75 suppliers
$30.00/5mg