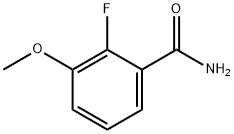
2-fluoro-3-MethoxybenzaMide synthesis
- Product Name:2-fluoro-3-MethoxybenzaMide
- CAS Number:198204-64-7
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15
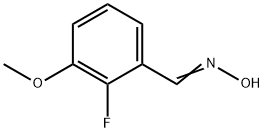
1143571-79-2
2 suppliers
inquiry

198204-64-7
9 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 2-fluoro-3-methoxybenzaldehyde oximewith sodium hydroxide in water; for 0.0333333 h;
Stage #2: with dihydrogen peroxide in water;
Steps:
Preparation of 13a and 13b
General procedure: The aldehydes 10a or 10b (800 mg, 5.2 mmol) were added to a solution of hydroxylamine hydrochloride (725 mg, 10.5 mmol) in DMSO (10 mL), and the reaction mixture was stirred at 100 C for 20 min. The heater was turned off and an aqueous solution of NaOH (600 mg) in H2O (5 mL) was slowly added to the reaction mixture over a 2 min period with stirring, and then 50% hydrogen peroxide (5 mL) was slowly and carefully added over a 10 min period. The reaction mixture was further stirred for 5 min and extracted with ethyl acetate (3 10 mL), dried over anhydrous MgSO4, and evaporated under reduced pressure to afford the corresponding amides 11a and 11b58 as white solids. The crude amides 11a or 11b (1 mmol) and Lawesson’s reagent (490 mg, 1.2 mmol) were added to dry THF (15 mL). The reaction mixture was stirred at room temperature for 3 h. The solvent was evaporated under reduced pressure and the residue was partitioned between aq NaHCO3 (25 mL) and ethyl acetate (25 mL). The organic solvent was separated and dried over anhydrous Na2SO4. The crude product was further purified by silica gel flash chromatography, using hexane-ethyl acetate (4:1), to yield the corresponding thioamides 12a and 12b as yellow solids. The obtained thioamides 12a or 12b (90 mg, 0.5 mmol), 3-methoxy-a-bromoacetophenone 8b (115 mg, 0.5 mmol), and potassium carbonate (175 mg, 0.5 mmol) were added to absolute ethanol (5 mL). The reaction mixture was heated at 70 C for 6 h and then allowed to cool and quenched with distilled water (30 mL). The organic materials were extracted by ethyl acetate (30 mL). The organic layer was isolated and dried over anhydrous Na2SO4. Solvent was evaporated under reduced pressure. The solid residue was purified by silica gel flash chromatography, using hexane-ethyl acetate (9:1, then 4:1), to yield thedesired compounds as solids
References:
Mayhoub, Abdelrahman S.;Marler, Laura;Kondratyuk, Tamara P.;Park, Eun-Jung;Pezzuto, John M.;Cushman, Mark [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 24,p. 7030 - 7039]
137654-20-7
160 suppliers
$8.00/1g

198204-64-7
9 suppliers
inquiry

850563-45-0
26 suppliers
$45.00/1g

198204-64-7
9 suppliers
inquiry
103438-88-6
160 suppliers
$14.14/250mgs:

198204-64-7
9 suppliers
inquiry
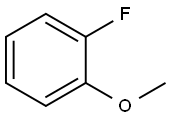
321-28-8
385 suppliers
$6.00/1g

198204-64-7
9 suppliers
inquiry