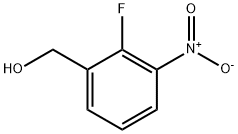
(2-Fluoro-3-nitrophenyl)methanol synthesis
- Product Name:(2-Fluoro-3-nitrophenyl)methanol
- CAS Number:946126-95-0
- Molecular formula:C7H6FNO3
- Molecular Weight:171.13
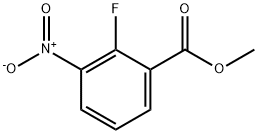
946126-94-9
160 suppliers
$28.00/1g

946126-95-0
67 suppliers
$48.00/250mg
Yield:946126-95-0 95%
Reaction Conditions:
Stage #1: 2-fluoro-3-nitrobenzoic acid methyl esterwith diisobutylaluminium hydride in toluene at -78 - 0; for 1 h;
Stage #2: with water;rochelle salt in methanol;toluene at -78 - 20; for 1 h;
Steps:
2; 3
Compound 2c-1: (2-Fluoro-3-nitrophenyl)methanol; [Show Image] [Show Image] DIBAL (115.7 mL, 1.0 M in toluene) was added at -78°C to a solution of 2-fluoro-3-nitrobenzoic acid methyl ester (compound 2b-1) (9.22 g, 46.3 mmol) in toluene (92 mL), and the reaction mixture was stirred at -78°C for 30 minutes and at 0°C for 30 minutes. The resultant reaction solution was cooled again to -78°C, and methanol, aqueous saturated Rochelle salt solution and ethyl acetate were added thereto. The reaction mixture was then stirred at room temperature for 1 hour, and extracted three times with ethyl acetate. The organic layer was washed with saturated saline and dried over magnesium sulfate. The title compound (7.52 g, 95%) was then obtained by vacuum concentration as a brown oil. 1H NMR (CDCl3) δ (ppm): 7.95 (m, 1H), 7.84 (t, 1H), 7.31 (t, 1H), 4.87 (s, 2H). HPLC Rt = 7.52 min. HPLC conditions were the same as those for the manufacturing example for compound 3a-1.
References:
EP1982982,2008,A1 Location in patent:Page/Page column 198
317-46-4
314 suppliers
$6.00/1g

946126-95-0
67 suppliers
$48.00/250mg
53553-14-3
81 suppliers
$14.00/100mg

946126-95-0
67 suppliers
$48.00/250mg
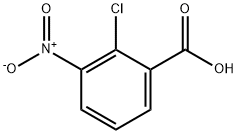
3970-35-2
268 suppliers
$6.00/250mg

946126-95-0
67 suppliers
$48.00/250mg