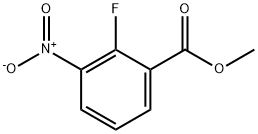
methyl 2-fluoro-3-nitrobenzoate synthesis
- Product Name:methyl 2-fluoro-3-nitrobenzoate
- CAS Number:946126-94-9
- Molecular formula:C8H6FNO4
- Molecular Weight:199.14
 A solution of 2-fluoro-3-nitrobenzoic acid (5 g, 27.0 mmol) in MeOH (50 mL) was treated with concentrated H2SO4 (1.4 mL, 27.0 mmol) and the resulting reaction was stirred at 50 °C for 16 h. The solvent was removed under reduced pressure and the residue was diluted with EtO Ac. The organic solution was washed with saturated aqueous NaHCO3 solution until the water phase reached neutral pH. The two phases were separated. Organics were washed with water, brine and dried (Na2SO4). The solution was filtered and concentrated to give crude methyl 2-fluoro-3-nitrobenzoate which was used as is in the next step. (5.1 g, 25.2 mmol, 93 %) as a pale yellow solid.
A solution of 2-fluoro-3-nitrobenzoic acid (5 g, 27.0 mmol) in MeOH (50 mL) was treated with concentrated H2SO4 (1.4 mL, 27.0 mmol) and the resulting reaction was stirred at 50 °C for 16 h. The solvent was removed under reduced pressure and the residue was diluted with EtO Ac. The organic solution was washed with saturated aqueous NaHCO3 solution until the water phase reached neutral pH. The two phases were separated. Organics were washed with water, brine and dried (Na2SO4). The solution was filtered and concentrated to give crude methyl 2-fluoro-3-nitrobenzoate which was used as is in the next step. (5.1 g, 25.2 mmol, 93 %) as a pale yellow solid.

67-56-1
738 suppliers
$9.00/25ml
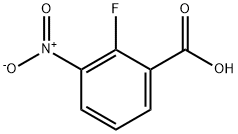
317-46-4
259 suppliers
$6.00/1g

946126-94-9
157 suppliers
$28.00/1g
Yield:946126-94-9 99%
Reaction Conditions:
Stage #1: 2-fluoro-3-nitro-benzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20;
Stage #2: methanol in dichloromethane; for 0.0833333 h;
Steps:
General procedure 3: Ester synthesis:
General procedure: To a suspension/solution of carboxylic acid (1.0 eq.) in dry DCM (0.5 M) was added oxalyl chloride (1.1 eq.) and DMF (0.05 eq.) and the mixture was stirred at room temperature until complete gas evolution. Excess MeOH was added and after 5 minutes the solvent was removed under reduced pressure and the product dried in vacuo and used without further purification.
References:
Kl?vekorn, Philip;Pfaffenrot, Bent;Juchum, Michael;Selig, Roland;Albrecht, Wolfgang;Zender, Lars;Laufer, Stefan A. [European Journal of Medicinal Chemistry,2021,vol. 210] Location in patent:supporting information
3970-35-2
252 suppliers
$6.00/250mg

946126-94-9
157 suppliers
$28.00/1g
53553-14-3
74 suppliers
$14.00/100mg

946126-94-9
157 suppliers
$28.00/1g
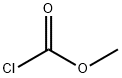
79-22-1
2 suppliers
$38.00/1000g

1493-27-2
506 suppliers
$5.00/10g

946126-94-9
157 suppliers
$28.00/1g
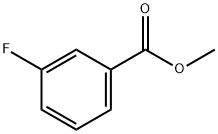
455-68-5
130 suppliers
$14.00/5g

946126-94-9
157 suppliers
$28.00/1g