
2-Fluoro-4-nitrobenzoic acid synthesis
- Product Name:2-Fluoro-4-nitrobenzoic acid
- CAS Number:403-24-7
- Molecular formula:C7H4FNO4
- Molecular Weight:185.11
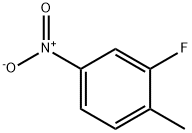
1427-07-2
354 suppliers
$6.00/5g

403-24-7
370 suppliers
$5.00/250mg
Yield:403-24-7 81%
Reaction Conditions:
with chromium(VI) oxide;periodic acid in acetonitrile; for 1 h;
Steps:
56
Periodic acid (1.69 g, 7.41 mmol) was dissolved in acetonitrile (25 mL) by vigorous stirring, and then chromium trioxide (0.16 g, 1.60 mmol) was dissolved into the solution. 2-Fluoro-4- nitrotoluene (0.33 g, 2.13 mmol) was added to the above solution with stirring. A white precipitate formed immediately with exothermic reaction. After 1 h of stirring, the supernatant liquid of the reaction mixture was decanted to a flask, and the solvent was removed by evaporation. The residues were extracted with methylene chloride (2x30 mL) and water (2x30 mL). The organic layer was dried over MgSO4, and concentrated to give 2-Fluoro-4-nitrobenzoic acid (Formula 37) (0.32 mg, 81%) as a white solid. 1HNMR δ 8.06 (ddd, 1 H, J=9.9, 2.2 and 0.3), 8.13 (ddd, 1 H, /=8.6, 2.2 and 0.9), 8.25 (ddd, 1 H,J=8.6, 7.0 and 0.3).
References:
WO2006/124118,2006,A1 Location in patent:Page/Page column 78
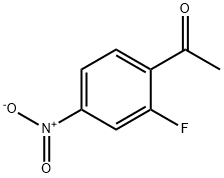
866579-96-6
26 suppliers
inquiry

403-24-7
370 suppliers
$5.00/250mg

1427-07-2
354 suppliers
$6.00/5g

64-19-7
1628 suppliers
$10.00/25ML

403-24-7
370 suppliers
$5.00/250mg