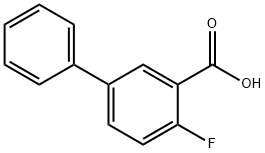
2-Fluoro-5-phenylbenzoic acid synthesis
- Product Name:2-Fluoro-5-phenylbenzoic acid
- CAS Number:146328-84-9
- Molecular formula:C13H9FO2
- Molecular Weight:216.21
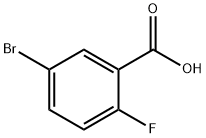
146328-85-0
277 suppliers
$6.00/1g

98-80-6
716 suppliers
$5.00/5g

146328-84-9
20 suppliers
$330.00/1g
Yield:146328-84-9 100%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);caesium carbonate in water;N,N-dimethyl-formamide at 110;Inert atmosphere;Suzuki Coupling;
Steps:
ee (ee) 4-Fluoro-f 1, 1 ‘biphenyl]-3-carboxylic acid (124)
To a degassed souton of DMF:H20 (10:1 raUo) (0.3 M), under an atmosphere of nitrogen, was added the pnaco ester (1 mmo), 2-bromopyrdne or 2-choropyrmdne (1 .5 eq.), and Cs2CO3 (4.4 eq.). The whoe mxture was degassed once agan and then Pd(PPh3)4 (5mo%) was added. The resutng souton was heated to 110°C overnght. The sovent was removed in vacuo to give a dark gummy residue, which was taken up nto EtOAc and H20, then acdfied with 2 M HC to pH 2. The organic ayer was separated and the aqueous ayer was further extracted with EtOAc (2x). The combined organic ayers were dried over MgSO4 and concentrated in vacuo to gve a back ofly residue. The residue was dry oaded ontosWca ge in vacuo then purfied by flash coumn chromatography, eutng with 10-30% EtOAc/petroeum benzne and 1% acetic acid to afford the tWe compound.acid was made as per genera “Suzuki Couphng F”,from 5-bromo-2-fluorobenzoc acid (1 g, 4.57 mmo) and phenythoronc acid (0.724 g, 5.94mmo), However after the reacUon mixture was coo’ed down t was passed through a bed of cehte, evaporated in-vacuo, acdfied with 1 M HC and the resuWng white precptate was ffltered off and dried n an oven at 130 °C (0.988 g, 100%). 1H NMR (400 MHz, DM80) 6 13.38 (s, 1H), 8.08 (dd, J= 7.0, 2.5 Hz, 1H), 7.93 (ddd, J= 8.5, 4.5, 2.6 Hz, 1H), 7.77-7.61 (m, 2H), 7.49 (dd, J= 10.3, 4.8 Hz, 2H), 7.41 (ddd, J= 7.8, 7.3, 5.9 Hz, 2H).
References:
WO2016/198507,2016,A1 Location in patent:Page/Page column 62

124-38-9
122 suppliers
$214.00/14L
324-74-3
178 suppliers
$15.00/1g

146328-84-9
20 suppliers
$330.00/1g
143-66-8
434 suppliers
$7.00/5g

146328-85-0
277 suppliers
$6.00/1g

146328-84-9
20 suppliers
$330.00/1g