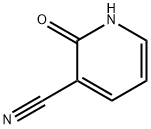
2-Hydroxy-3-cyanopyridine synthesis
- Product Name:2-Hydroxy-3-cyanopyridine
- CAS Number:20577-27-9
- Molecular formula:C6H4N2O
- Molecular Weight:120.11
6602-54-6
509 suppliers
$6.00/10g

20577-27-9
166 suppliers
$14.00/1g
Yield:20577-27-9 100%
Reaction Conditions:
with acetic acid for 12 h;Reflux;
Steps:
2-Bromo-3-cyanopyridine(24).
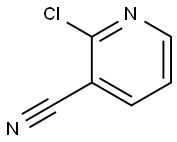
2-Chloro-3-cyanopyridine 23 (3.68 mg, 26 mmol) was boiled underreflux with AcOH (100 mL) for 12 h. The evaporation residue, in THF (80 mL)and H2O (20 mL), was boiled under reflux for 4 h. Evaporation gave 3-cyanopyridin-2-one(3.12 g, quant.) as white crystals: mp 110-114°C (lit.13174-175°C); 1H NMR d 6.47 (1 H, t, J =6.6 Hz, 5-H), 7.91 (1 H, dd, J = 6.5, 2.1 Hz, 4-H), 8.14 (1 H, dd, J =7.0, 2.1 Hz, 6-H), 11.48 (1 H, s, 1-H); 13C NMR d 105.80 (3-C), 124.05(5-C), 141.87 (CN), 144.50 (6-H), 150.02 (4-C), 154.20 (2-C); MS m/z 143.0214(M + Na)+ (C6H4N2NaO requires143.0222). Bu4NBr (8.55 g, 26 mmol) and P2O5(3.67 g, 26 mmol) were heated in PhMe (250 mL) at 80°C for 30 min. The above3-cyanopyridin-2-one (1.59 g, 13 mmol) was added and the mixture was boiledunder reflux for 12 h. The mixture was cooled, poured into cold water andextracted (EtOAc, 2 ×). Drying and evaporation gave 24 (2.24 g, 94%) asoff-white crystals.
References:
Kumpan, Katerina;Nathubhai, Amit;Zhang, Chenlu;Wood, Pauline J.;Lloyd, Matthew D.;Thompson, Andrew S.;Haikarainen, Teemu;Lehti?, Lari;Threadgill, Michael D. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 13,art. no. 12299,p. 3013 - 3032] Location in patent:supporting information