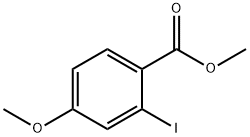
2-Iodo-4-methoxy-benzoic acid methyl ester synthesis
- Product Name:2-Iodo-4-methoxy-benzoic acid methyl ester
- CAS Number:54413-84-2
- Molecular formula:C9H9IO3
- Molecular Weight:292.07
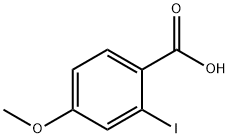
54435-09-5
82 suppliers
$11.00/100mg

74-88-4
341 suppliers
$15.00/10g

54413-84-2
30 suppliers
$326.00/5g
Yield:54413-84-2 60%
Reaction Conditions:
with 1,8-diazabicyclo[5.4.0]undec-7-ene in acetonitrile at 20;Inert atmosphere;
Steps:
4.1.10. Methyl-2-iodo-4-methoxybenzoate ( 10 ) [25]
A dry and argon-flushed round bottom flask equipped with a magnetic stirrer and a septum was charged with a solution of 9 (5 g, 18 mmol, 1 equiv.) in dry acetonitrile (40 mL). Iodomethane (1.46 mL, 23.4 mmol, 1.3 equiv.) and DBU (3.95 mL, 19.8 mmol, 1.1 equiv.) were successively added and the reaction mixture was stirred overnight at room temperature. The solvent was removed and the residue was diluted in ethyl acetate (50 mL). The organic phase was washed with water (3 ×50 mL), a aqueous solution of HCl 1 M (2 ×50 mL), a aqeous saturated solution of NaHCO 3 (2 ×50 mL) and brine (50 mL). The organic phase was the dried over MgSO 4 , filtered and solvent was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel using petroleum ether/ethyl acetate (95:5) as eluent to afford 10 (3.11 g, yield = 60%) as clear oil. 1 H NMR (300 MHz, CDCl 3 ) δ= 7.85 (d, J = 8.8 Hz, 1H), 7.52 (d, J = 2.5 Hz, 1H), 6.90 (dd, J = 8.8, 2.6 Hz, 1H), 3.89 (s, 3H), 3.83 (s, 3H). 13 C NMR (300 MHz, CDCl 3 ) δ 166.0, 162.0, 132.5, 131.6, 127.0, 113.6, 95.6, 55.6, 52.1.
References:
Ahmed, Elhadi;Camiade, Emilie;Chaar, Capucine;Fouquenet, Delphine;Hervé, Virginie;Ibraheem, Walaa;Petrignet, Julien;Roux, Anne-Emmanuelle;Si-Tahar, Mustapha;Thibonnet, Jér?me;Thiery, Emilie [Journal of Molecular Structure,2022,vol. 1252,art. no. 132135]

54435-09-5
82 suppliers
$11.00/100mg

54413-84-2
30 suppliers
$326.00/5g
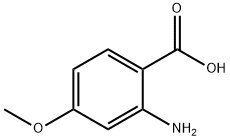
4294-95-5
241 suppliers
$14.00/1g

54413-84-2
30 suppliers
$326.00/5g