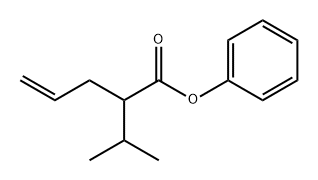
2-isopropylpent-4-enoic acid synthesis
- Product Name:2-isopropylpent-4-enoic acid
- CAS Number:1575-71-9
- Molecular formula:C8H14O2
- Molecular Weight:142.2
Yield:1575-71-9 86%
Reaction Conditions:
Stage #1: 3-methylbutyric acidwith lithium diisopropyl amide in tetrahydrofuran at -15; for 1 h;
Stage #2: allyl bromide in tetrahydrofuran at 20; for 1 h;
Steps:
1.1 Synthesis of 2-isopropyl-4-valeric acid
Add 15 mL of tetrahydrofuran to a 100 mL Leica bottle.10 mL of a solution of 2.0 mol/L lithium diisopropylamide in tetrahydrofuran, and then 1.1 mL (10.0 mmol) of isovaleric acid represented by Formula A-1 was added dropwise at -15°C, and the reaction was conducted for 1 hour, followed by addition of 1.04 mL ( 12.0 mmol) allyl bromide, naturally return to room temperature, continue the reaction for 1 hour, with a mass fraction of 10% hydrochloric acid to adjust the pH value of 1-2, 20 mL of petroleum ether was added for extraction, and then were respectively used 15mL mass fraction of 10% hydrochloric acid, The mixture was washed with water and brine, and the organic layers were combined and dried over anhydrous sodium sulfate. The organic solvent was removed under reduced pressure to obtain 1.2 g of 2-isopropyl-4-enovaleric acid represented by the formula D in a yield of 86%. as follows:
References:
CN107501129,2017,A Location in patent:Paragraph 0027-0030
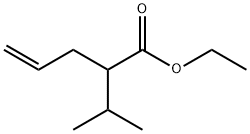
21244-42-8
0 suppliers
inquiry

1575-71-9
24 suppliers
inquiry
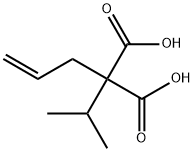
4375-26-2
3 suppliers
inquiry

1575-71-9
24 suppliers
inquiry

2835-39-4
45 suppliers
$7126.11/1KG
1195-42-2
66 suppliers
$12.00/250mg

1575-71-9
24 suppliers
inquiry