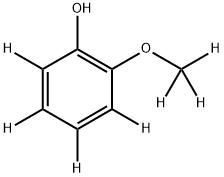
2-Methoxy-d3-phenol--d4 synthesis
- Product Name:2-Methoxy-d3-phenol--d4
- CAS Number:1065473-05-3
- Molecular formula:C7H8O2
- Molecular Weight:124.14
865-50-9
136 suppliers
$25.00/1g

103963-58-2
30 suppliers
$175.00/10mg

1065473-05-3
12 suppliers
$820.00/0.25G
Yield:-
Reaction Conditions:
Stage #1: 3,4,5,6-<2H4>-1,2-dihydroxybenzenewith 3,4-dihydro-2H-pyran;pyridinium p-toluenesulfonate in dichloromethane at 20; for 3 h;
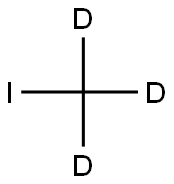
Stage #2: iodomethane-d3with potassium carbonate in acetone at 40; for 24 h;Inert atmosphere;
Steps:
1
(Intermediate 7) (2R)-2-[({2-[(2H3)Methoxy]-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) (2H3)phenyl}oxy)methyl}-1,4-dioxane (Step 1) 4-Bromo-2,3,5-trideuterio-6-(trideuteriomethoxy)phenol 4-bromo-2-[(2H3)methoxy] (2H3)phenol (2H4)Benzene-1,2-diol (12.0 g, 105 mmol) was suspended in dichloromethane (105 ml). To the suspension, 3,4-dihydro-2H-pyran (9.53 ml, 105 mmol) and p-toluenesulfonic acid pyridine salt (396 mg, 1.58 mmol) were added, and the mixture was stirred at room temperature for 3 hours. To the reaction mixture, a saturated aqueous solution of sodium bicarbonate was added, and the mixture was stirred for 10 minutes. The organic layer was washed with brine and dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The obtained oil was dissolved in acetone (207 ml). To the solution, potassium carbonate (45.8 g, 330 mmol) and trideuteriomethyl iodide (14.4 ml, 228 mmol) were added, and the mixture was heated with stirring at 40° C. for 24 hours under nitrogen stream. The reaction mixture was cooled to room temperature and then filtered with a Kiriyama funnel, and the filtrate was concentrated under reduced pressure. To the residue, hexane was added, and the mixture was filtered again with a Kiriyama funnel. Then, the filtrate was concentrated under reduced pressure. The obtained oil was dissolved in ethanol (181 ml). To the solution, p-toluenesulfonic acid pyridine salt (341 mg, 1.36 mmol) was added, and the mixture was heated with stirring at 65° C. for 3 hours. The reaction mixture was cooled to room temperature and then concentrated under reduced pressure. The residue was dissolved in N,N-dimethylformamide (90.0 ml). To the solution, N-bromosuccinimide (16.1 g, 90.6 mmol) was added in small portions under ice cooling, and the mixture was stirred for 1 hour under ice cooling. To the reaction mixture, diethyl ether (500 ml) was added, and then, the reaction was terminated by the addition of an 8.85 mol/L aqueous sodium thiosulfate solution (50 ml). The organic layer was dried over anhydrous magnesium sulfate and then concentrated under reduced pressure. The residue was purified by amino silica gel column chromatography (Shoko Scientific Co., Ltd., elution solvent: hexane/ethyl acetate=90/10->55/45) to obtain the title compound (6.97 g, yield: 28.3%) as an oil. 1H-NMR (CDCl3) δ: 5.57 (1H, s). MS (CI) m/z: 207 [(M-H)+].
References:
US2017/183329,2017,A1 Location in patent:Paragraph 0409-0412