
2-METHOXYPHENYLBORONIC ACID PINACOL ESTER synthesis
- Product Name:2-METHOXYPHENYLBORONIC ACID PINACOL ESTER
- CAS Number:190788-60-4
- Molecular formula:C13H19BO3
- Molecular Weight:234.1
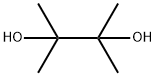
76-09-5
377 suppliers
$8.19/250mg
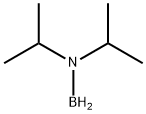
22092-92-8
4 suppliers
inquiry
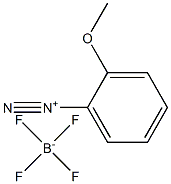
492-95-5
1 suppliers
inquiry

190788-60-4
103 suppliers
$5.00/250mg
Yield: 69%
Reaction Conditions:
Stage #1:diisopropopylaminoborane;2-methoxyphenyldiazonium tetrafluoroborate with ferrocene in acetonitrile at 20; for 2.5 h;
Stage #2: with methanol in acetonitrile at 0 - 20; for 1 h;
Stage #3:2,3-dimethyl-2,3-butane diol in diethyl ether;acetonitrile at 20; for 4 h;Reagent/catalyst;
Steps:
5 Example 5: synthesis of 2-( -methoxyphenyr)-4,4,5,5-tetramethyl-l,3,2-dioxaborolane [CAS 190788-60-41], compound VIA2
Example 3: General procedure D for the synthesis of the arylpinacolboronates by arylation of diisopropylaminoborane, catalysed by ferrocene (1%), followed by methanolysis and transesterification In a dried tube reactor under argon as described in example 2, the arenediazonium salt (1 mmol) and the ferrocene (ΙΟμιηοΙ, 1.8mg) were dissolved in 2mL of anhydrous CH3CN. Diz'sopropylaminoborane (2mmol, 226mg) was then added to the solution and the mixture was stirred for 2h30 at room temperature. The reaction mixture was quenched by a slow addition of anhydrous MeOH at 0°C (2mL) and stirred for an additional hour at room temperature. After removal of all the volatiles, 1.3eq of pinacol was added in Et20 (2mL), the mixture was stirred 4h at room temperature. The crude mixture was washed with a 50g/L CuCl2 solution (2 x 5mL). The organic layer were separated, dried over Na2S04, filtered and concentrated to dryness. The resulted oil was dissolved with CH2C12 and filtered of a pad of silica gel, eluting with CH2C12 to afford the corresponding boronate. Example 5: synthesis of 2-( -methoxyphenyr)-4,4,5,5-tetramethyl-l,3,2-dioxaborolane iCAS 190788-60-41, compound VI 161 mg of 2-(l-methoxyphenyl)-4,4,5,5-tetramethyl-l,3,2-dioxaborolane were obtained following the general procedure D according to example 3, using 222 mg of 2- methoxybenzenediazonium tetrafluoroborate as a pale yellow oil, with an isolated yield of 69%. 1H NMR (300 MHz, CDC13) δ 7.67 (dd, J = 7.3, 1.8 Hz, 1H), 7.39 (ddd, J = 8.5, 7.5, 1.9 Hz, 1H), 6.94 (t, J = 7.3 Hz, 1H), 6.86 (d, J = 8.4 Hz, 1H), 3.83 (s, 3H), 1.36 (s, 12H) nB NMR (100 MHz, CDC13) δ 31.09 13C NMR (75 MHz, CDC13) δ 136.69, 132.43, 120.21, 110.51, 83.45, 55.84, 24.84
References:
UNIVERSITE DE BORDEAUX 1;CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE;PUCHEAULT, Mathieu Jonathan Damien;MARCIASINI, Ludovic Daniel Alain;VAULTIER, Michel WO2014/9169, 2014, A1 Location in patent:Page/Page column 54-55
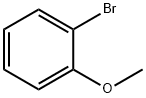
578-57-4
302 suppliers
$6.00/10g

73183-34-3
564 suppliers
$6.00/5g

190788-60-4
103 suppliers
$5.00/250mg

529-28-2
240 suppliers
$8.00/5g

73183-34-3
564 suppliers
$6.00/5g

190788-60-4
103 suppliers
$5.00/250mg
766-51-8
206 suppliers
$20.00/25g

73183-34-3
564 suppliers
$6.00/5g

190788-60-4
103 suppliers
$5.00/250mg
100-66-3
512 suppliers
$10.00/5G

73183-34-3
564 suppliers
$6.00/5g
171364-79-7
139 suppliers
$6.00/1g

190788-60-4
103 suppliers
$5.00/250mg
325142-84-5
112 suppliers
$6.00/1g