
2-Methoxyquinoline-7-carboxylic acid synthesis
- Product Name:2-Methoxyquinoline-7-carboxylic acid
- CAS Number:1374258-40-8
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
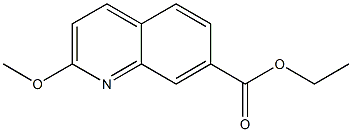
1374258-39-5
4 suppliers
inquiry

1374258-40-8
11 suppliers
inquiry
Yield:1374258-40-8 96%
Reaction Conditions:
Stage #1: ethyl 2-methoxyquinoline-7-carboxylatewith water;lithium hydroxide in tetrahydrofuran at 20; for 65 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; pH=4;
Steps:
19.3
To a solution of ethyl 2-methoxyquinoline-7-carboxylate (125 mg, 0.54 mmol) in tetrahydrofuran (1.5 mL) was added 2 N aqueous lithium hydroxide (0.81 mL, 1.6 mmol). The reaction was stirred at room temperature for 65 hours. The tetrahydrofuran was removed in vacuo and the residue was acidified to pH=4 with 1 N aqueous hydrochloric acid. The mixture was diluted with water and the resulting precipitate was collected by filtration and dried under vacuum to give the title compound (106 mg, 96%) as a white solid. +ESI (M+H) 204.2; 1H NMR (400 MHz, CDCl3, δ): 8.64 (d, J=1.37 Hz, 1H), 8.01-8.04 (m, 1H), 8.01 (s, 1 H), 7.79 (d, J=8.58 Hz, 1H), 7.01 (d, J=8.78 Hz, 1H), 4.09 (s, 3H).
References:
US2012/108619,2012,A1 Location in patent:Page/Page column 36

1451154-40-7
16 suppliers
inquiry

1374258-40-8
11 suppliers
inquiry
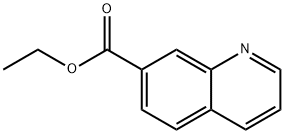
104294-00-0
24 suppliers
inquiry

1374258-40-8
11 suppliers
inquiry
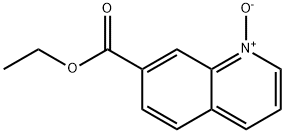
1355191-89-7
1 suppliers
inquiry

1374258-40-8
11 suppliers
inquiry

51552-68-2
49 suppliers
$41.00/100mg

1374258-40-8
11 suppliers
inquiry