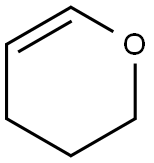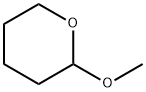
2-METHOXYTETRAHYDROPYRAN synthesis
- Product Name:2-METHOXYTETRAHYDROPYRAN
- CAS Number:6581-66-4
- Molecular formula:C6H12O2
- Molecular Weight:116.16
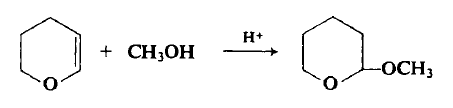
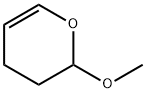
4454-05-1
133 suppliers
$9.00/25g

6581-66-4
62 suppliers
$21.00/1g
Yield: 96%
Reaction Conditions:
with hydrogen;palladium on activated carbon at 20; under 6000.6 - 15001.5 Torr; for 1.5 h;Product distribution / selectivity;
Steps:
2; 3; 8
Example 2:; In a 100 mL-volume autoclave made of stainless steel, 23.17 g of 3,4-dihydro-2-methoxy-2H-pyran(DHMP) and 0.42 g of 5 mass % palladium/activated carbon powder(Pd/C) were placed. The reactor was purged with hydrogen, and 0.8 MPa of hydrogen was introduced. This was stirred at a room temperature and hydrogen was continuously introduced into the reactor so that the pressure 0.8 MPa was maintained during the reaction. In this step, the amount of the hydrogen gas introduced into the reactor was 4737 ml(Step-l) . After 1.5 hour, DHMP as raw material was not detected. Tetrahydro-2-methoxy-2H-pyran(THMP) was produced at a yield of 96 %. After adding 1.10 g of sodium hydrogen sulfate hydrate to the reaction mixture, the pH of the reaction mixture measured was 1 (Step-2) . As in Step-1, the reactor was pressurized to 0.8 MPa with hydrogen. Reaction was performed at 70°C for 2 hours, 1000C for 2 hours, and then 130 0C for 1.5 hours, while continuously introducing hydrogen into the reactor. In this step, the amount of the hydrogen gas introduced into the reactor was 3989 ml(Step-3) .As a result of the reaction mixture after the reaction, the yield of tetrahydropyran(THP) was 82 %. THMP was generated at a yield of 1 % and methanol was generated at a yield of 80 % based on the amount of the raw material.; Example 3:; The same procedures were carried out as in Example 2 except that 23.79 g of 3, 4-dihydro-2-methoxy-2H-pyran(DHMP) and 0.21 g of 5 mass % palladium/activated carbon powder(Pd/C) were used and that 0.38 g of p-toluene sulfonic acid monohydrate was EPO
References:
SHOWA DENKO K.K. WO2006/62211, 2006, A1 Location in patent:Page/Page column 21-24

4454-05-1
133 suppliers
$9.00/25g
142-68-7
184 suppliers
$13.00/25mL

6581-66-4
62 suppliers
$21.00/1g

67-56-1
739 suppliers
$7.29/5ml-f

67-56-1
739 suppliers
$7.29/5ml-f
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6581-66-4
62 suppliers
$21.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
5324-21-0
0 suppliers
inquiry

6581-66-4
62 suppliers
$21.00/1g