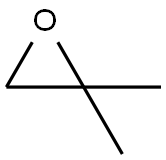
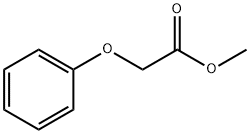
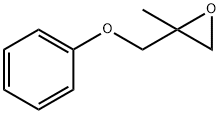
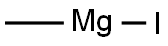2-methyl-1-phenoxypropan-2-ol synthesis
- Product Name:2-methyl-1-phenoxypropan-2-ol
- CAS Number:13524-74-8
- Molecular formula:C10H14O2
- Molecular Weight:166.22

621-87-4
106 suppliers
$43.50/1g

13524-74-8
6 suppliers
inquiry
Yield:13524-74-8 2.1 g (91% recovery)
Reaction Conditions:
with methylmagnesium bromide in diethyl ether;
Steps:
26 1,1-Dimethyl-2-phenoxyethanol
PREPARATION 26 1,1-Dimethyl-2-phenoxyethanol To a stirred solution of 24.2 g (0.16 mole) of phenoxy-2-propanone (Eastman) in 150 mL of dry ethyl ether was added 56 ml (0.17 mole) of methylmagnesium bromide (3.0M solution in ethyl ether, Aldrich) and the reaction mixture was stirred at ambient temperature under a nitrogen atmosphere for 4 hr. The reaction mixture was treated with 100 ml of saturated ammonium chloride solution and vigorously stirred for 1 hr. The layers were separated and the organic layer was washed twice with 200 ml portions of water, dried (magnesium sulfate) and the solvent evaporated under reduced pressure to yield 24.3 g (91%) of a vicous oil. A 2.3 g sample of the oil was purified by high pressure liquid chromatography (Waters Associates Prep LC/System 500 A, PrepPak 500 silica, ethyl acetate-hexanes, 1:20, flow rate 150 ml/min). The desired fractions were combined and the solvents evaporated under reduced pressure to yield 2.1 g (91% recovery) of the title compound as a colorless liquid. Analysis: Calculated for C10 H14 O2: C, 72.26; H, 8.49. Found: C, 72.08; H, 8.46.
References:
US5192785,1993,A
15895-57-5
1 suppliers
inquiry

13524-74-8
6 suppliers
inquiry