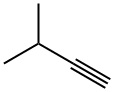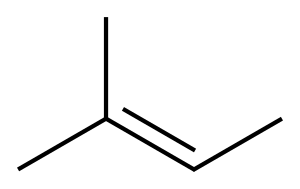
2-Methyl-2-butene synthesis
- Product Name:2-Methyl-2-butene
- CAS Number:513-35-9
- Molecular formula:C5H10
- Molecular Weight:70.13
Yield:-
Reaction Conditions:
with Pb-poisoned 5% Pd-CaCO3;hydrogen at 74.84; under 750.075 Torr;chemoselective reaction;
Steps:
2. Experimental
The gas-phase hydrogenation of valylene (VL) (ABCR-Chemicals, purity 97%), isoprene (IP) (ABCR-Chemicals, purity 99%), 3-methylbutyne (3MBy) (Acros Organics, purity 96%), and 1-penten-4-yne (ABCR-Chemicals, purity 95%) was studied at 1 bar in a fully-automated set-up equipped with mass-flow controllers (Bronkhorst, EL-FLOW) to feed gases (H2, CO, and He), a syringe pump (Chemyx, Fusion 100) to feed the liquid hydrocarbons, an electrically-heated oven with a 12 mm i.d. quartz micro-reactor,and a gas chromatograph. The catalysts (sieve fraction 200-400 lm) wereloaded in the tubular reactor and heated in He at 573 K for 30 min. Pd and PdAPb were evaluated as such, while the other catalysts were subjected to an additional pre-treatment: PdACO (0.1 vol.% CO in He, 348 K, 10 min), Cu, Ni, and CuANi(5 vol.% H2 in He, 773 K, 30 min). The liquid hydrocarbon was evaporated at 353 K and mixed with the gas mixture prior to the reactor inlet. The hydrogenation reactions were carried out at 348 K (Pd, PdACO, PdAPb), and 523 K (Cu, Ni, CuANi). Reaction temperatures and pre-treatment conditions were selected on the basis of previous tests over these specific catalysts in propyne hydrogenation [37]. The hydrogen-to-hydrocarbon ratio dependence and 3-methylbutyne and 1-penten-4-yne hydrogenation were carried out over 0.3 g of catalyst. The inlet hydrocarbon concentration was 2.5 vol.%, the inlet H2 concentration varied in the range of 2.5-30 vol.%, and the total flow was kept at 84 cm3 STP min-1 by balancing the feed mixtures with He. The influence of the degree of hydrocarbon conversion on the product distribution was studied using catalyst amounts in the range of 0.01-0.2 g. In the experiments with a weight of catalyst 60.1 g, the bed was diluted with SiC (sieve fraction 200-400 lm) to minimize by-passing. The inlet hydrocarbon concentration was 2.5 vol.%, the inlet H2 concentration was 2.5 vol.% (Pd, PdAPb) and 5 vol.% (PdACO, Cu, Ni, CuANi). The total flow was varied in the range of 20-270 cm3 STP min-1, yielding space velocities (SV) of 6000-1620,000 cm3 g-1 h-1. High space velocities were necessary in order to attain a low degree ofconversion on the palladium-based catalysts. Hydrogenation tests with Pd/c-Al2O3 were also carried out in the presence of 0.1 vol.% CO. Fresh catalyst was loaded for each hydrocarbon substrate, while the hydrogen-to-hydrocarbon and conversion dependence were studied without changing the catalytic bed. The catalytic performance was measured in steady state, after equilibration for at least 1 h at each experimental condition. The catalysts were stableduring time-on-stream which went up to 10 h and this was corroborated by running the same experimental condition in different stages. Additionally, tests with different particle sizes and different total volumetric flows (at constant space velocity) were conducted to discard internal and external mass-transport constraints. It was further emphasized by the fulfillment of the Weisz-Prater criterion. Products in the outlet stream were analyzed using an on-line gas chromatograph (Agilent GC7890A) equipped with a GS-GasPro column (and an additional HP-Plot Q for the separation of the1-penten-4-yne hydrogenation products) and a flame ionization detector. The tubes from the syringe connection to the gas chromatograph were heated at 393 K to prevent reactants and products condensation. The conversion, X, was determined as the amount of converted hydrocarbon divided by the amount of hydrocarbon atthe reactor inlet.
References:
Bridier, Blaise;Perez-Ramirez, Javier [Journal of Catalysis,2011,vol. 284,# 2,p. 165 - 175] Location in patent:experimental part

123-51-3
553 suppliers
$25.00/25mL

513-35-9
187 suppliers
$12.00/25g
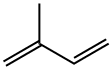
78-79-5
263 suppliers
$14.56/25ML

513-35-9
187 suppliers
$12.00/25g
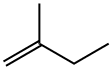
563-46-2
87 suppliers
$40.00/5G
563-45-1
132 suppliers
$15.00/5G

78-79-5
263 suppliers
$14.56/25ML

513-35-9
187 suppliers
$12.00/25g
78-78-4
281 suppliers
$21.00/25ML

563-46-2
87 suppliers
$40.00/5G
75-85-4
358 suppliers
$9.00/10ml

513-35-9
187 suppliers
$12.00/25g