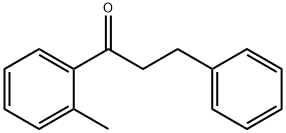
2'-METHYL-3-PHENYLPROPIOPHENONE synthesis
- Product Name:2'-METHYL-3-PHENYLPROPIOPHENONE
- CAS Number:93433-65-9
- Molecular formula:C16H16O
- Molecular Weight:224.3
Yield:93433-65-9 94.8%
Reaction Conditions:
with chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II);1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine;1,4-di(diphenylphosphino)-butane;bis-[(trifluoroacetoxy)iodo]benzene in N,N-dimethyl-formamide at 80; for 11 h;Reagent/catalyst;Solvent;
Steps:
3 Example 3
To a solution of N, N-dimethylformamide (DMF) in a volume ratio of 4: 1 with polyethylene glycol 200(PEG-200)) was added 100 mmol of the compound of the above formula (I), 60 mmol of the compound of the above formula (II), 8 mmol of catalyst bis (triphenylphosphine) cyclopentadienyl ruthenium chloride, 120 mmol of an oxidizing agent bis Fluoroacetic acid) iodobenzene (PhI (TFA) 2),15 mmol of organic ligands L1 and 80 mmol of base, 5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) were added and the mixture was allowed to warm to 80 ° C with stirring and the reaction was stirred at that temperature for 11 hours; After completion of the reaction, the reaction system is naturally cooled to room temperature and the pH is adjusted to neutral, filtered and the filtrate is saturatedWashed with brine and extracted with ethyl acetate 2-3 times. The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography at a 1: 2 by volume mixture of chloroform and petroleum ether Followed by elution to give the compound of formula (III) in a yield of 94.8%.
References:
CN106278839,2017,A Location in patent:Paragraph 0051; 0052; 0053; 0054; 0055

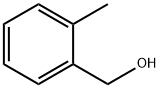
100-51-6
1384 suppliers
$5.00/100g
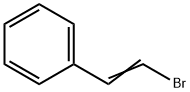
122382-54-1
0 suppliers
inquiry

93433-65-9
10 suppliers
$433.00/1g
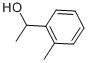
7287-82-3
68 suppliers
$49.89/5g

100-51-6
1384 suppliers
$5.00/100g

93433-65-9
10 suppliers
$433.00/1g
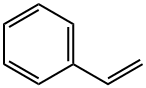
100-42-5
619 suppliers
$20.00/25mL
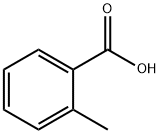
118-90-1
491 suppliers
$13.00/1g

93433-65-9
10 suppliers
$433.00/1g
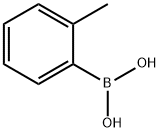
16419-60-6
372 suppliers
$9.00/1g
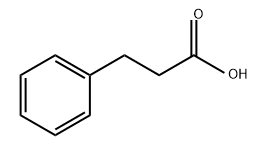
501-52-0
484 suppliers
$14.14/1gm:

93433-65-9
10 suppliers
$433.00/1g