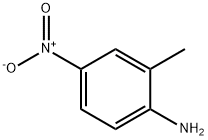
2-Methyl-6-nitroaniline synthesis
- Product Name:2-Methyl-6-nitroaniline
- CAS Number:570-24-1
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15
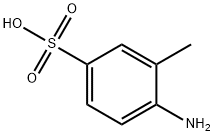
98-33-9
192 suppliers
$6.00/25g

570-24-1
347 suppliers
$6.00/10g
Yield:570-24-1 82%
Reaction Conditions:
Stage #1:2-aminotoluene-5-sulfonic acid with acetic acid;zinc(II) oxide at 80; for 4 h;
Stage #2: with nitric acid at 10 - 12; for 2 h;
Stage #3: with hydrogenchloride in water at 100; for 1 h;
Steps:
1-3 Example 1
Under mechanical stirring, 60.1g (1.00mol) acetic acid was dropped into a mixture of 93.6g (0.50mol) 4-amino-3-methylbenzenesulfonic acid and 4.2g (0.05mol) zinc oxide. After the addition, The temperature was raised to 80°C for 4h. After the reaction is complete, filter through a pad of diatomaceous earth, transfer the filtrate to an ice water bath, and cool to 10-12°C. Add 63ml of concentrated nitric acid (1.00mol HNO3) dropwise, the addition is completed in about 1.5h, the temperature is controlled at 10-12°C during the dropping, and the reaction is maintained at 10-12°C for 30 minutes after the dropping. After nitrification is complete, The reaction solution was poured into 1.5L ice water, and suction filtered to obtain the nitration product solid (without drying). Put the nitration product solid in the flask, add 150ml concentrated hydrochloric acid (1.80mol HCl), React at 100°C for 1h. After the hydrolysis is completed, it is cooled to room temperature, 150 ml of water is added, a solid is separated out, filtered with suction, and the filter cake is dried to obtain 62.1 g of an orange solid with a yield of 82.0% and a purity of 98.2%.
References:
Zhejiang University of Technology;Zhang Guofu;Xu Yingbiao;Li Yongshu CN112159325, 2021, A Location in patent:Paragraph 0015; 0020-0025
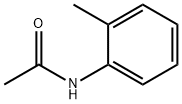
120-66-1
149 suppliers
$10.00/5g

570-24-1
347 suppliers
$6.00/10g
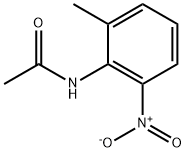
59907-22-1
25 suppliers
$33.39/250mg

570-24-1
347 suppliers
$6.00/10g

59907-22-1
25 suppliers
$33.39/250mg

570-24-1
347 suppliers
$6.00/10g
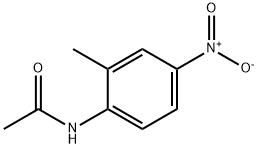
2719-15-5
39 suppliers
$65.90/5g