
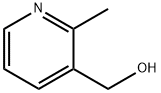
(2-METHYL-PYRIDIN-3-YL)-METHANOL synthesis
- Product Name:(2-METHYL-PYRIDIN-3-YL)-METHANOL
- CAS Number:76915-53-2
- Molecular formula:C7H8BrN
- Molecular Weight:186.05
56826-61-0
102 suppliers
$143.00/1g

76915-53-2
31 suppliers
$50.00/10mg
Yield:76915-53-2 3.52 g
Reaction Conditions:
with phosphorus tribromide in dichloromethane at 0 - 20; for 1.5 h;
Steps:
b Step b:
To a 0 °C mixture of(2-methylpyridin-3-yl) methanol (1.41 g, 11.45 mmol) in DCM (20 mL) was added PBr3 (1.86 g, 6.87 mmol) dropwise. The resulting mixture was allowed to warm to RT and stirred for 1.5 h. The reaction mixture was taken to pH 8 using aq. NaOH (5 M, 10 mL). The aqueous layer was separated and the organic layer was washed with brine (1 x 20 mL), dried over anhydrous Na2504 and concentrated under reduced pressure to give 3-(bromomethyl)-2-methylpyridine as a yellow oil (3.52 g) which was used in next step without any further purification. MS: m/z 186 (M+H) .
References:
WO2018/172984,2018,A1 Location in patent:Page/Page column 66
583-61-9
340 suppliers
$5.00/5g

76915-53-2
31 suppliers
$50.00/10mg
1721-26-2
178 suppliers
$15.00/1g

76915-53-2
31 suppliers
$50.00/10mg
3222-56-8
230 suppliers
$8.00/1g

76915-53-2
31 suppliers
$50.00/10mg