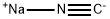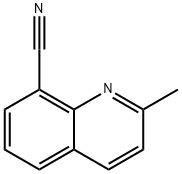
2-Methylquinoline-8-carbonitrile synthesis
- Product Name:2-Methylquinoline-8-carbonitrile
- CAS Number:864779-05-5
- Molecular formula:C11H8N2
- Molecular Weight:168.19
Yield:864779-05-5 98%
Reaction Conditions:
with copper(l) iodide;tetrakis(triphenylphosphine) palladium(0) in acetonitrile; for 2 h;Inert atmosphere;Reflux;
Steps:
88.B
To a solution of 2- methylquinolin-8-yl trifluoromethanesulfonate (3.0 g, 10.3 mmol) in acetonitrile (26 mL) was added sodium cyanide (1.0 g, 20.6 mmol). The solution was degassed under nitrogen for 10 minutes, followed by addition of copper (I) iodide (0.20 g, 1.03 mmol) and Pd(PPh3)4 (0.60 g, 0.52 mmol) under nitrogen. The mixture was heated at reflux for 2 hours. After cooling the mixture was diluted with ethyl acetate (50 mL) and filtered through Celite and washed with ethyl acetate (50 mL). The filtrate was washed with water and brine, dried (MgS04), filtered and concentrated under reduced pressure. The residue was purified by column chromatography (Biotage, 40 M; 20% ethyl acetate: hexanes) to afford 2-methylquinoline-8- carbonitrile (1.70 g, 98%).
References:
WO2012/154274,2012,A1 Location in patent:Page/Page column 120

826-81-3
273 suppliers
$11.00/25g

864779-05-5
19 suppliers
inquiry
61047-43-6
104 suppliers
$60.00/100mg
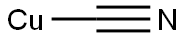
544-92-3
248 suppliers
$9.00/1g

864779-05-5
19 suppliers
inquiry