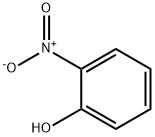
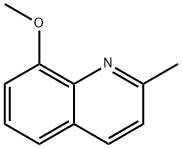
8-Hydroxyquinaldine synthesis
- Product Name:8-Hydroxyquinaldine
- CAS Number:826-81-3
- Molecular formula:C10H9NO
- Molecular Weight:159.18
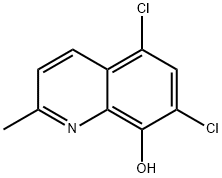
3033-80-5
93 suppliers
$26.00/250mg

826-81-3
273 suppliers
$11.00/25g
Yield:826-81-3 76%
Reaction Conditions:
with hydrogen bromide for 36 h;Inert atmosphere;Reflux;
Steps:
1. General procedure for synthesis of 8-hydroxyquinolines 6a and 15
General procedure: To a 50 mL round-bottom flask was sequentially added o-anisidine (1.13 mL, 10.0 mmol), 3.6 mL concentrated sulfuric acid, and 15 milligrams sodium iodide and the reaction was then heated to 110°C. The respective enone/enal reagent (1.7 equivalents, 16.6 mmol) was added dropwise over 2 hours and allowed to react for an additional 4 hours. After that time, the reaction was allowed to cool to room temperature, and transferred to a separatory funnel containing 62 mL of an aqueous 1 M solution of sodium carbonate. The mixture was then extracted with dichloromethane (5 x 80 mL) and the organic layers were combined and further extracted with 12 M hydrochloric acid (5 x 50 mL). The resulting aqueous extracts were combined and neutralized with 8 M sodium hydroxide (350 mL) before being extracted with dichloromethane. The organic layers were combined and dried with anhydrous sodium sulfate, filtered and concentrated in vacuo to afford the crude product which was taken on to the next step without purification. To a 100 mL round-bottom flask was added crude methoxyquinoline intermediates (9 mmol) and 18 mL of concentrated (i.e., 48 %) hydrobromic acid. The reaction was heated to reflux for 36 hours. After this time, the reaction was allowed to cool to room temperature, transferred to a separatory funnel where the reaction was neutralized with 8 M sodium hydroxide and extracted with dichloromethane. The organic extracts were combined, filtered and concentrated in vacuo and passed through a 1-inch plug of silica gel (eluting with dichloromethane). Solvent was removed in vacuo to afford 6a (33%, 71.6 milligrams, white solid) and 15 (76%, 1.09 g, light brown solid).
References:
Abouelhassan, Yasmeen;Garrison, Aaron T.;Burch, Gena M.;Wong, Wilson;Norwood, Verrill M.;Huigens, Robert W. [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 21,p. 5076 - 5080] Location in patent:supporting information
72-80-0
186 suppliers
$5.00/100mg

826-81-3
273 suppliers
$11.00/25g
1004545-76-9
0 suppliers
inquiry

826-81-3
273 suppliers
$11.00/25g