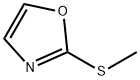
2-methylthiooxazole synthesis
- Product Name:2-methylthiooxazole
- CAS Number:201017-90-5
- Molecular formula:C4H5NOS
- Molecular Weight:115.15
32091-51-3
11 suppliers
$778.00/1g

74-88-4
344 suppliers
$15.00/10g

201017-90-5
23 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium carbonate in acetonitrile at 25; for 6 h;Inert atmosphere;
Steps:
4.a; 64.a
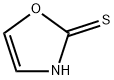
a) Preparation of 2-(methylsulfonyl)-1,3-oxazole Under an atmosphere of protective gas, 1,3-oxazole-2(3H)-thione (1.00 g, 10 mmol; prepared according to WO 03/006442 A) is initially charged in 20 ml of acetonitrile. Iodomethane (1.544 g, 0.677 ml, 11 mmol) is added dropwise, followed by potassium carbonate (1.503 g, 11 mmol). The mixture is stirred at 25° C. for 6 hours. For work-up, the reaction mixture is added to water and extracted twice with dichloromethane (100 ml), and the extract is then washed with water and finally with saturated NaCl solution. The combined organic phases are dried over magnesium sulfate, filtered off and directly reacted further. With stirring and ice-cooling, 3-chloro-perbenzoic acid (5.100 g, 23 mmol, 77% pure) is then added a little at a time to the resultung dichloromethane solution, and the mixture is stirred at 25° C. for a further 6 hours and then allowed to stand overnight. For work-up, the reaction mixture is washed twice with 2-molar sodium hydroxide solution, then with water and finally with saturated NaCl solution. The combined organic phases are dried over magnesium sulfate, filtered off and concentrated. This gives 0.820 g of product (50.7% of theory). NMR (CDCl3, 400 MHz): 3.35 (s, 3H, CH3); 7.38 (br s, 1H); 7.88 (br s, 1H).
References:
US2011/190125,2011,A1 Location in patent:Page/Page column 25

32091-51-3
11 suppliers
$778.00/1g

74-88-4
344 suppliers
$15.00/10g

201017-90-5
23 suppliers
inquiry