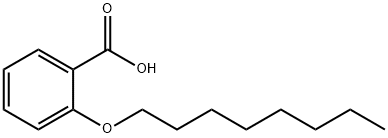
2-n-Octyloxybenzoic acid synthesis
- Product Name:2-n-Octyloxybenzoic acid
- CAS Number:27830-12-2
- Molecular formula:C15H22O3
- Molecular Weight:250.33
255062-85-2
21 suppliers
inquiry

27830-12-2
20 suppliers
inquiry
Yield:27830-12-2 97%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;methanol;water at 20 - 85;
Steps:
1.B [0116] General Procedure- Step B: hydrolysis of methyl 2-(octyloxy)benzoate (4).
A slurry of the methyl 2-(octyloxy)benzoate (8.6 g, 32.8 mmol) in MeOH/THF (v:v = 1:1, 40 ml) at room temperature was treated with NaOH (1M solution in H2O, 100 ml, 100 mmol). The reaction mixture was heated to 85°C for overnight. Removal of the solvent in vacuo followed by dissolving the residue solids with H2O and acidified with 1N HCl, and extracted with EtOAc (3x 100 mL). The combined organic extracts were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude material was purified by Biotage flash chromatography (gradient elution, 0 to 30% EtOAc in hexanes) to obtain the title compound 4 (8.0 g, 97%) as colorless oil.1H NMR (400MHz, DMSO-d6) d 12.31 (s, 1H), 7.59 (dd, J = 7.6, 1.8 Hz, 1H), 7.46 (ddd, J = 7.8, 7.8, 1.8 Hz, 1H), 7.09 (d, J = 8.2 Hz, 1H), 6.96 (dd, J = 7.6, 7.6 Hz, 1H), 4.01 (t, J = 6.4 Hz, 1H), 1.70 (m, 2H), 1.43 (m, 2H), 1.35- 1.22 (m, 8H), 0.86 (t, J = 6.8 Hz, 3H). LCMS (m/z) 251.2 (M+H); RT (Method B, std LCMS method), 1.34 min
References:
WO2020/163479,2020,A1 Location in patent:Paragraph 0116

111-83-1
491 suppliers
$12.00/25g

27830-12-2
20 suppliers
inquiry
119-36-8
992 suppliers
$5.00/10g

27830-12-2
20 suppliers
inquiry