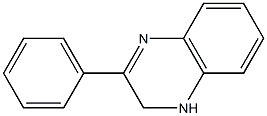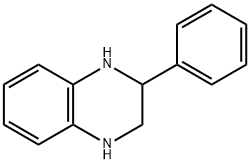
2-PHENYL-1,2,3,4-TETRAHYDRO-QUINOXALINE synthesis
- Product Name:2-PHENYL-1,2,3,4-TETRAHYDRO-QUINOXALINE
- CAS Number:5021-47-6
- Molecular formula:C14H14N2
- Molecular Weight:210.27
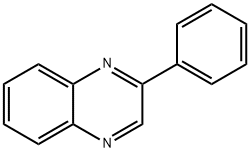
5021-43-2
24 suppliers
inquiry

5021-47-6
14 suppliers
$59.60/250mg
Yield:5021-47-6 97%
Reaction Conditions:
Stage #1: AG 1152with bis[dichlorido(η5-1,2,3,4,5-pentamethyl-cyclopentadienyl)iridium(III)];N-(2-aminoethyl)-4-(trifluoromethyl)benzenesulfonamide;anhydrous sodium formate;anhydrous Sodium acetate;glacial acetic acid in lithium hydroxide monohydrate at 80; pH=4.3; for 10 h;
Stage #2: with potassium hydroxide in lithium hydroxide monohydrate;regioselective reaction;
Steps:
4.4. General procedure for transfer hydrogenation of quinoxalines
General procedure: A carousel reaction tube containing a magnetic stirring bar and [Cp*IrCl2]2 (2 mg, 2.5 μmol), ligand 3d (1.6 mg, 6 μmol), quinoxaline substrate (0.5 mmol), and HCOONa (340 mg, 5 mmol) in an aqueous solution of HOAc/NaOAc (5 M, 5 mL, pH=5.5) was sealed without degassing. The reaction mixture was stirred at 80 °C for the time indicated in Table 3, then cooled to room temperature and basified with an aqueous solution of KOH. The resulting mixture was extracted with diethyl ether (3×5 mL) and dried over Na2SO4. The solvent was removed under reduced pressure, and the product was purified by flash column chromatography.
References:
Tan, Jing;Tang, Weijun;Sun, Yawei;Jiang, Zhen;Chen, Fei;Xu, Lijin;Fan, Qinghua;Xiao, Jianliang [Tetrahedron,2011,vol. 67,# 34,p. 6206 - 6213] Location in patent:experimental part

1074-12-0
109 suppliers
$48.00/1g
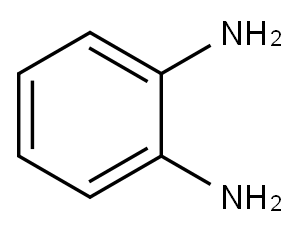
95-54-5
526 suppliers
$9.00/1g

5021-47-6
14 suppliers
$59.60/250mg
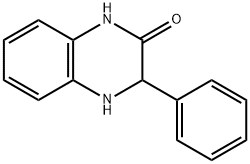
23465-73-8
1 suppliers
inquiry

5021-47-6
14 suppliers
$59.60/250mg
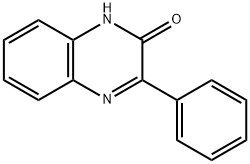
1504-78-5
32 suppliers
$125.00/1g

5021-47-6
14 suppliers
$59.60/250mg