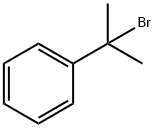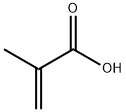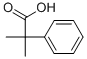
2-Phenylisobutyric acid synthesis
- Product Name:2-Phenylisobutyric acid
- CAS Number:826-55-1
- Molecular formula:C10H12O2
- Molecular Weight:164.2
201230-82-2
1 suppliers
inquiry
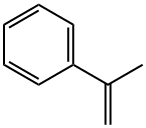
98-83-9
294 suppliers
$10.00/25mL

826-55-1
292 suppliers
$6.00/10g
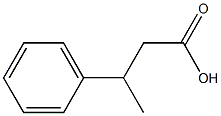
772-17-8
1 suppliers
inquiry
Yield:-
Reaction Conditions:
with triethylsilane;water;[((S)-(+)-3,5-bis(di(xylyl)phosphino)-[2.2]-paracyclophane)dipalladium] tetrachloride;toluene-4-sulfonic acid;lithium chloride in butanone at 90; under 22502.3 Torr;Sealed vial;Inert atmosphere;Autoclave;
Steps:
12
Example 12: Hydroxycarbonylation of alpha-methyl-styrene using [Pd2(Xyl- Phanephos)CI4]LiCI (4.2 mg, 0.1 mmol), p-TsOH (17.2 mg, 0.1 mmol) and [(Xyl-(S)- Phanephos)Pd2CI4] (0.005 mmol) were weighed into a 5 ml microwave vial. A magnetic stirrer bar was added, the vial was sealed with a crimp cap and put under an inert atmosphere, a-methylstyrene (65 μΙ, 0.5 mmol), water (22.5 μΙ, 1.25 mmol) and butanone (1.5 ml) were then added by syringe, as was the internal standard Et4Si (23.5μΙ, 0.125 mmol). The caps were pierced with two needles and the vials placed into an autoclave, under an inert atmosphere, and the autoclave quickly sealed. The autoclave was purged three times with CO, then pressurised to 30 bar and heated in a preheated oil bath to 90 °C. After 20 hours the autoclave was cooled to room temperature and the pressure released slowly. A sample of the crude product was removed for analysis by 1H NMR spectroscopy. The solvent was removed from the remaining reaction mixture under vacuum and the residue dissolved in toluene. The product was extracted 3 times with saturated NaHC03 solution and the combined aqueous phases were acidified with cone. HCI. The solution was then extracted 3 times with EtOAc. The combined organic layers were dried over MgS04, filtered and the solvent removed under vacuum, to give the carboxylic acid product as a 4:94 mixture of quaternary and linear isomers (84% conversion, 77 % isolated yield).
References:
WO2011/30110,2011,A2 Location in patent:Page/Page column 29-30
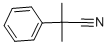
1195-98-8
97 suppliers
inquiry

826-55-1
292 suppliers
$6.00/10g