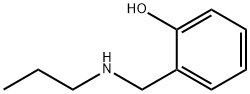
2-[(propylamino)methyl]phenol synthesis
- Product Name:2-[(propylamino)methyl]phenol
- CAS Number:84672-90-2
- Molecular formula:C10H15NO
- Molecular Weight:165.23
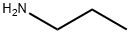
107-10-8
354 suppliers
$10.00/20mg

90-02-8
445 suppliers
$9.00/1g
![2-[(propylamino)methyl]phenol](/CAS/20180808/GIF/84672-90-2.gif)
84672-90-2
13 suppliers
$45.00/25mg
Yield:84672-90-2 55%
Reaction Conditions:
Stage #1: propylamine;salicylaldehyde in methanol at 0 - 20; for 3.5 h;
Stage #2: with sodium tetrahydroborate in methanol at 0 - 20;
Steps:
N-(Substituted benzyl) propan-1-amine (1a-o)
General procedure: The appropriate benzaldehydes (3.33 mmol) was dissolved in anhydrous methanol (20 ml) and cooled to 0 °C under stirring. To this cold mixture of n-propylamine (0.39 ml, 6.66 mmol) was added dropwise and stirring was continued for 30 min at 0 °C, then for 3 hr at ambient temp. The reaction mixture was again cooled to 0 °C and NaBH4 (0.19 g, 5.0 mmol) was added in three portions at 10 min intervals. Then it was stirred at room temp for another 1 hr and the solvent was distilled to 1/3 of its original volume under vacuum. Cold water (5 ml) and NaHCO3 (0.28 g, 3.33 mmol) were added to the above concentrated solution, stirred for 10 min and extracted with methylene chloride (3 x 20 ml). Combined organic phases was dried over Na2SO4 and evaporated the solvent and dried under vacuum to get the compounds (1a-o).
References:
Srivastav, Maneesh Kumar;Rajeeva;Salahuddin, Md.;Srinivasulu;Shanta Kumar [Indian Journal of Heterocyclic Chemistry,2014,vol. 24,# 2,p. 115 - 118]
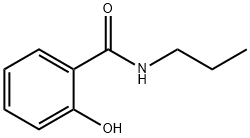
7461-94-1
2 suppliers
inquiry
![2-[(propylamino)methyl]phenol](/CAS/20180808/GIF/84672-90-2.gif)
84672-90-2
13 suppliers
$45.00/25mg
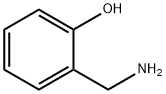
932-30-9
161 suppliers
$10.00/250mg
![2-[(propylamino)methyl]phenol](/CAS/20180808/GIF/84672-90-2.gif)
84672-90-2
13 suppliers
$45.00/25mg

90-02-8
445 suppliers
$9.00/1g
![2-[(propylamino)methyl]phenol](/CAS/20180808/GIF/84672-90-2.gif)
84672-90-2
13 suppliers
$45.00/25mg
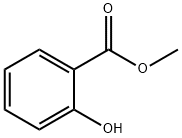
119-36-8
988 suppliers
$5.00/10g
![2-[(propylamino)methyl]phenol](/CAS/20180808/GIF/84672-90-2.gif)
84672-90-2
13 suppliers
$45.00/25mg