
2-PYRIDIN-2-YL-BENZOOXAZOL-5-YLAMINE synthesis
- Product Name:2-PYRIDIN-2-YL-BENZOOXAZOL-5-YLAMINE
- CAS Number:61431-37-6
- Molecular formula:C12H9N3O
- Molecular Weight:211.22
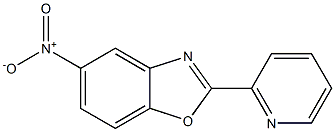
61382-18-1
0 suppliers
inquiry

61431-37-6
19 suppliers
$354.00/1g
Yield:61431-37-6 1.94 g
Reaction Conditions:
with hydrazine in ethanol at 60;
Steps:
3.1.1. General Synthesis of 3a-3c
General procedure: 5-Nitro-2-(pyridin-2-yl)benzo[d]oxazole (3a). To a solution of 2-picolinic acid (2.46 g0.01 mol) condensed with 4-nitro-2-aminophenol (3.08 g, 0.01 mol) in polyphosphoric acid(30 mL) in a 100mL three-necked flask, the middle neck was installed a mechanical stirring,and the side neck was set for thermometer. The reaction mixture was heated 140 C and theexternal temperature was kept at 150 C. After 6 h, the reaction was completed through TLC.Then the reaction mixture was poured into water slowly and was adjusted to pH 6 usingpotassium carbonate. A quantity of solid precipitated and was collected by suction. Thiscompound was used without further purification and drying. Because the next reductionwas carried out in ethyl alcohol, the crude product 2a was used directly. The crude 2a wasdissolved in 95% ethyl alcohol (40 mL), and then was addedW-2 Raney nickel (ca. 0.20 g),and then hydrazine (1.96 g, 3 equiv.) was introduced drop wise. Moreover, the mixturewas heated to 60 C and monitored by TLC. After completion, the reaction mixture wasfiltered through a Celite pad. The filtration was condensed and purified by flash columnchromatography (petroleum ether: ethyl acetate) to obtain 3a (1.94 g, 72% for two steps) asa brown solid:
References:
Wang, Ruibo;Kang, Ruiting;Yang, Xuan;Cheng, Yu;Bai, Hongjin;Du, Zhenting [Molecules,2022,vol. 27,# 23,art. no. 8375]

99-57-0
9 suppliers
$22.00/25g

61431-37-6
19 suppliers
$354.00/1g
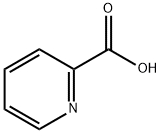
98-98-6
602 suppliers
$5.00/10g

61431-37-6
19 suppliers
$354.00/1g
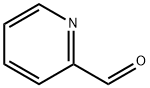
1121-60-4
505 suppliers
$10.60/10gm:

61431-37-6
19 suppliers
$354.00/1g