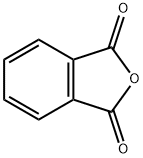
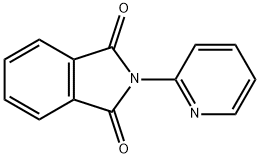
2-pyridin-3-ylisoindole-1,3-dione synthesis
- Product Name:2-pyridin-3-ylisoindole-1,3-dione
- CAS Number:19171-27-8
- Molecular formula:C13H8N2O2
- Molecular Weight:224.21
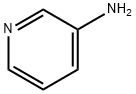
462-08-8
470 suppliers
$14.00/25g

88-67-5
535 suppliers
$5.00/5g
201230-82-2
1 suppliers
inquiry

19171-27-8
6 suppliers
inquiry
Yield:19171-27-8 81%
Reaction Conditions:
with triethylamine in toluene at 100; under 760.051 Torr; for 4 h;Autoclave;
Steps:
General procedure for carbonylative cyclization of2-iodobenzoic acid for the synthesis of N-substitutedisoindole-1,3-diones using ImmPd-IL as a catalyst
General procedure: To a 100 mL stainless steel autoclave, 2-iodobenzoic acid(1 mmol), aryl amine (2 mmol), ImmPd-IL (2 mol%), toluene (10 mL)and Et3N (2.5 mmol) were added. The autoclave was closed, purgedthree times with nitrogen followed with carbon monoxide andthen pressurized with 1 atm of CO and heated at 100C for 4 h.After completion of reaction the reactor was cooled to room tem-perature, the remaining CO gas was carefully vented, and thereactor was opened. The reactor vessel was thoroughly washedwith ethyl acetate (10-15 mL) to remove any traces of productand catalyst if present. The catalyst was filtered and the reac-tion mixture was evaporated under vacuum. The residue obtainedwas purified by column chromatography (silica gel, 60-120 mesh;petroleum ether/ethyl acetate, 95:05) to afford the desired prod-uct. The products were confirmed by GC, GC-MS,1H NMR and13C NMR spectroscopic techniques. The purity of compounds wasdetermined by GC-MS analysis.
References:
Khedkar, Mayur V.;Shinde, Ajinkya R.;Sasaki, Takehiko;Bhanage, Bhalchandra M. [Journal of Molecular Catalysis A: Chemical,2014,vol. 385,p. 91 - 97]

462-08-8
470 suppliers
$14.00/25g
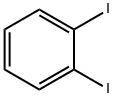
615-42-9
280 suppliers
$6.00/1g
201230-82-2
1 suppliers
inquiry

19171-27-8
6 suppliers
inquiry

462-08-8
470 suppliers
$14.00/25g
201230-82-2
1 suppliers
inquiry
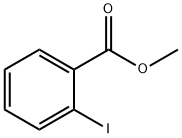
610-97-9
408 suppliers
$9.00/1g

19171-27-8
6 suppliers
inquiry
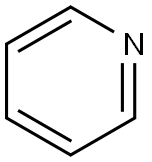
110-86-1
843 suppliers
$9.00/5g
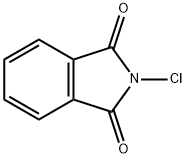
3481-09-2
165 suppliers
$11.00/5g
59208-49-0
12 suppliers
inquiry

19171-27-8
6 suppliers
inquiry