
2-Pyridinecarboxaldehyde, 6-ethoxy- (9CI) synthesis
- Product Name:2-Pyridinecarboxaldehyde, 6-ethoxy- (9CI)
- CAS Number:85259-47-8
- Molecular formula:C8H9NO2
- Molecular Weight:151.17
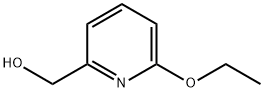
890655-75-1
11 suppliers
inquiry

85259-47-8
27 suppliers
inquiry
Yield:85259-47-8 70%
Reaction Conditions:
Stage #1: (6-ethoxypyridin-2-yl)methanolwith oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -60 - -20; for 3.16667 h;
Stage #2: with triethylamine in dichloromethane at 20; for 0.666667 h;
Steps:
6.c
DMSO (0.50 mL, 6.4 mmol) in CH2Cl2 (10 mL) was added dropwise to a solution of oxalyl chloride (2M in CH2Cl2, 3.1 mL, 6.1 mmol) in CH2Cl2 (20 mL) at -60 °C. The resulting mixture was stirred at -60 0C for 10 minutes. (6-Ethoxy-pyridin-2-yl)-methanol (0.85 g, 5.6 mmol) in CH2Cl2 (5 mL) and DMSO (4 mL) was added dropwise. The mixture was stirred at -60 0C for 3 h, and was then allowed to warm to -20 °C and Et3N (6 mL) was added. The resulting solution was stirred at ambient temperature for 40 minutes. Water was added and the mixture was extracted with CH2Cl2. The organic phase was washed with brine, dried (Na2SO4), and concentrated. Diethyl ether was added to the residue and EPO
References:
WO2006/62465,2006,A1 Location in patent:Page/Page column 38-39
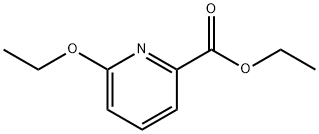
890655-74-0
10 suppliers
inquiry

85259-47-8
27 suppliers
inquiry
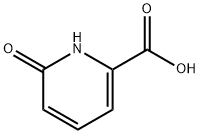
19621-92-2
230 suppliers
$7.00/1g

85259-47-8
27 suppliers
inquiry