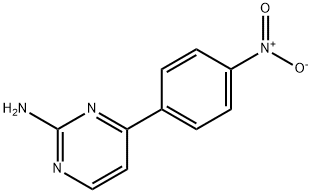
2-PyriMidinaMine, 4-(4-nitrophenyl)- synthesis
- Product Name:2-PyriMidinaMine, 4-(4-nitrophenyl)-
- CAS Number:99361-84-9
- Molecular formula:C10H8N4O2
- Molecular Weight:216.2
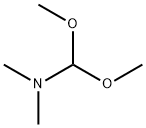
78089-99-3
24 suppliers
$45.00/100mg
50-01-1
742 suppliers
$5.00/25g

99361-84-9
32 suppliers
inquiry
Yield:99361-84-9 97.3%
Reaction Conditions:
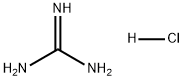
Stage #1: guanidine hydrochloridewith potassium carbonate in ethanol at 20; for 0.666667 h;
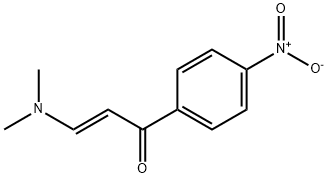
Stage #2: (E)-3-dimethylamino-1-(4-nitrophenyl)-2-propen-1-one in ethanol at 80; for 20 h;
Steps:
8.2 Preparation of 4-(4-Nitrophenyl)-2-aminopyrimidine(Intermediate 3b)
Take guanidine hydrochloride (34.06mmol, 3.35g) and add ethanol (20ml) into 250ml dry two-necked flask.After the addition, potassium carbonate (51.09 mmol, 7.06 g) was added and the mixture was stirred at room temperature for 40 min. Intermediate 2b (11.35 mmol, 2.50 g) was added and the mixture was warmed to 80° C. and refluxed for 20 h. The reaction was completed by TLC and cooled to room temperature. The solvent was evaporated under reduced pressure, washed with water, filtered and dried to give a yellow solid.
References:
CN104447706,2017,B Location in patent:Paragraph 0089-0090
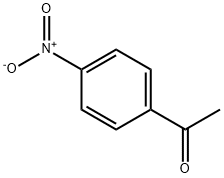
100-19-6
305 suppliers
$5.00/5g

99361-84-9
32 suppliers
inquiry