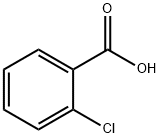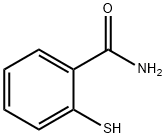
2-sulfanylbenzamide synthesis
- Product Name:2-sulfanylbenzamide
- CAS Number:5697-20-1
- Molecular formula:C7H7NOS
- Molecular Weight:153.2
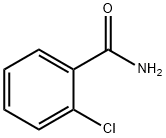
609-66-5
244 suppliers
$10.00/5g

5697-20-1
10 suppliers
inquiry
Yield:5697-20-1 88%
Reaction Conditions:
with sodium sulfide in 1-methyl-pyrrolidin-2-one;water at 130 - 190; for 4 h;Inert atmosphere;Temperature;
Steps:
2
23.4 g (0.18 mol) of 60% sodium sulfide (40% water) and 160 g N-methyl-2-pyrrolidone (NMP) was added to a 500 ml neck flask provided with a heated oil bath, stirrer and a thermometer. The mixture was stirred at 190° C. and purged with nitrogen to a weight loss of 25 g. To the dried slurry of sodium sulfide at 130° C., 18.1 g (0.1163 mol), 2-chlorobenzamide of 98% purity was added and the mixture heated to 175° C. for 4 hours. HPLC analysis of the reaction mixture, calibrated with authentic samples showed (g; mol; molar % of theory): (0.37; 0.0012; 2) 2,2′-dithiodibenzoic acid and (16.5; 0.108; 88) 2-mercaptobenzamide. 2-chlorobenzoic acid was not detected. [0033] The mixture was cooled to 70° C., 40 g of water was added and pH adjusted to 4.0, by adding 28.5 g of 35% hydrochloric acid. The mixture was heated to boiling until hydrogen sulfide evolution ceased. Evolved hydrogen sulfide was absorbed for disposal in a caustic solution. At 20° C., caustic solution (such as alkali hydroxide or carbonates or other salts or other bases) was added to the reaction mixture to bring the pH back to 9 or above and 27.0 g (0.111 mol) 14% hydrogen peroxide was introduced over 30 min. [0034] Water and NMP was distilled off at reduced pressure and the residue was dispersed in 125 g of water. The mixture was adjusted to pH 5 with 35% hydrochloric acid. Separated BIT crystals were filtered off, washed with water and air dried to constant weight. BIT yield 14.3 g (0.094 mol) which is 80.8% of theory. Purity 99.5% by HPLC. The NMP can be recovered for reuse by known methods.
References:
US2014/316141,2014,A1 Location in patent:Paragraph 0032-0034
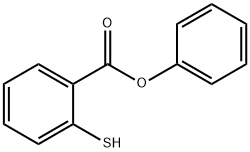
15570-18-0
2 suppliers
inquiry

5697-20-1
10 suppliers
inquiry

2527-57-3
7 suppliers
inquiry

5697-20-1
10 suppliers
inquiry
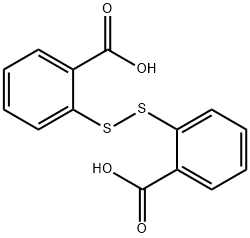
119-80-2
425 suppliers
$7.00/25g

5697-20-1
10 suppliers
inquiry