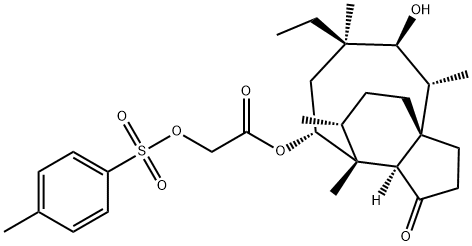
2’-Tosyloxy-dihydropleuromutilin synthesis
- Product Name:2’-Tosyloxy-dihydropleuromutilin
- CAS Number:933762-22-2
- Molecular formula:C29H42O7S
- Molecular Weight:534.7

31716-01-5
28 suppliers
$1450.00/50mg

933762-22-2
11 suppliers
$80.00/10mg
Yield:933762-22-2 96.2%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in tetrahydrofuran at 25; under 2068.65 Torr; for 12 h;
Steps:
1
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7- vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (500.0 mg, 938.6 umol, 1.0 eq) and Pd/C (300.0 mg, 938.6 umol, 1.0 eq) in THF (30.0 mL) were stirred at 25°C for 12 hours under 40 psi hydrogen atmosphere. The mixture was filtered and the filtrate was concentrated to give (3aR,4R,5R,7R,8S,9R,9aS,12R)- 7-ethyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a- propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (500.0 mg, 902.8 umol, 96.2% yield, 96.5% purity) as white foam. XH NMR (CHLOROFORM-d, 400MHz) δ 7.83 (d, J = 8.4 Hz, 2H), 7.36 (d, J= 8.4 Hz, 2H), 5.66 (d, J = 7.9 Hz, 1H), 4.51 (s, 2H), 3.45-3.37 (m, 1H), 2.46 (s, 3H), 2.41-2.14 (m, 4H), 2.09 (br. s., 1H), 1.85-1.05 (m, 15H), 0.99-0.91 (m, 6H), 0.72 (t, J = 7.5 Hz, 3H), 0.61 (d, J = 7.1 Hz, 2H).
References:
WO2017/151492,2017,A1 Location in patent:Paragraph 0259

98-59-9
585 suppliers
$9.00/5g

933762-22-2
11 suppliers
$80.00/10mg