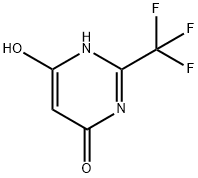
2-(TRIFLUOROMETHYL)PYRIMIDINE-4,6-DIOL synthesis
- Product Name:2-(TRIFLUOROMETHYL)PYRIMIDINE-4,6-DIOL
- CAS Number:672-47-9
- Molecular formula:C5H3F3N2O2
- Molecular Weight:180.08
108-13-4
266 suppliers
$9.00/1g
383-63-1
479 suppliers
$10.00/25 g

672-47-9
72 suppliers
$45.00/50mg
Yield:672-47-9 44.4%
Reaction Conditions:
with NaH in toluene;mineral oil;butan-1-ol
Steps:
3 4,6-Dihydroxy-2-trifluoromethylpyrimidine, Compound VI, starting material for Step 2
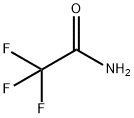
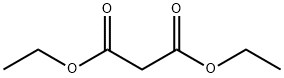
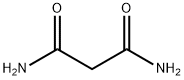
EXAMPLE 3 4,6-Dihydroxy-2-trifluoromethylpyrimidine, Compound VI, starting material for Step 2 Sodium hydride (900 g, 57.5% dispersion in mineral oil; 518 g active NaH; 22.5M) was stirred with 7.5 L toluene in a 22 L round-bottomed flask. Butanol was added over 5 hr. so that the pot temperature was maintained at 40°. The mixture was stirred an additional 16 hr. Malonamide (765 g; 7.5 M) was added, followed by ethyl trifluoroacetate (1065 g; 7.5 M). The ensuing reaction was exothermic; the mixture was then heated on a steam-bath for 3.5 hrs. It was then stirred at 23°-25° for an additional 16 hrs. The mixture was extracted with water (1*4 L and 1*2 L). The combined aqueous extracts were treated with activated charcoal and filtered. The filtrate was maintained at 10°-15° as it was acidified to pH 1-2 with 37% hydrochloric acid. The mixture was chilled to 5°. The solid was isolated by filtration and dried at 50° in vacuo to give 600 g (44.4% yield) VI m.p. 255°-256° (Lit. 265°).
References:
Bristol-Myers Squibb Co. US4963678, 1990, A