2-[(trifluoromethyl)sulfanyl]phenol synthesis
- Product Name:2-[(trifluoromethyl)sulfanyl]phenol
- CAS Number:33185-56-7
- Molecular formula:C7H5F3OS
- Molecular Weight:194.17
Yield:33185-56-7 57%
Reaction Conditions:
with sodium dihydrosulfite;triethylamine in lithium hydroxide monohydrate;acetonitrile at -20 - 25;Inert atmosphere;
Steps:
4.2. General procedure
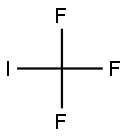
General procedure: Acetonitrile (150 mL), thiophenol (25 g, 227 mmol, 1 equiv.) andtriethylamine (25.3 g, 250 mmol, 1.1 equiv.) were placed in the flasksuccessively under stirring. The reaction mixture was cooled to -20 Cand the argon atmosphere was pumped out. A rubber balloon chargedwith CF3I (49 g, 250 mmol, 1.1 equiv.) was joined to the flask by a plug.After absorption of CF3I by the reaction solution, the flask was filled withan argon atmosphere. Sodium dithionite (39.5 g, 227 mmol, 1 equiv.)solution in H2O (100 mL) was added dropwise. Some self-heating forabout 1 to 30 C of the reaction mixture could be observed. Afterreaching ambient temperature (25 C) the reaction was judged completeand the mixture was ready for aqueous work up.
References:
Orlova, Raisa K.;Sokolenko, Liubov V.;Babadzhanova, Lesia A.;Filatov, Andrey A.;Yagupolskii, Yurii L. [Journal of Fluorine Chemistry,2022,vol. 261-262,art. no. 110004]
887144-97-0
163 suppliers
$28.00/250mg
1121-24-0
148 suppliers
$26.00/5G
![2-[(trifluoromethyl)sulfanyl]phenol](/CAS/20210111/GIF/33185-56-7.gif)
33185-56-7
7 suppliers
inquiry
456-56-4
145 suppliers
$13.00/1g
![2-[(trifluoromethyl)sulfanyl]phenol](/CAS/20210111/GIF/33185-56-7.gif)
33185-56-7
7 suppliers
inquiry