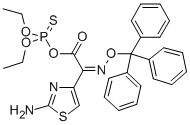
4-THIAZOLEACETIC ACID, 2-AMINO-ALPHA-[(TRIPHENYLMETHOXY)IMINO]-, ANHYDRIDE WITH O,O-DIETHYL HYDROGEN PHOSPHOROTHIOATE, (Z)- synthesis
- Product Name:4-THIAZOLEACETIC ACID, 2-AMINO-ALPHA-[(TRIPHENYLMETHOXY)IMINO]-, ANHYDRIDE WITH O,O-DIETHYL HYDROGEN PHOSPHOROTHIOATE, (Z)-
- CAS Number:200814-98-8
- Molecular formula:C28H28N3O5PS2
- Molecular Weight:581.64

2524-04-1
7 suppliers
$16.80/25G
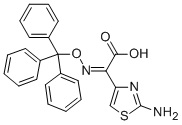
128438-01-7
75 suppliers
$45.00/50mg
![4-THIAZOLEACETIC ACID, 2-AMINO-ALPHA-[(TRIPHENYLMETHOXY)IMINO]-, ANHYDRIDE WITH O,O-DIETHYL HYDROGEN PHOSPHOROTHIOATE, (Z)-](/CAS/GIF/200814-98-8.gif)
200814-98-8
19 suppliers
inquiry
Yield:200814-98-8 89%
Reaction Conditions:
Stage #1: (Z)-2-(2-aminothiazol-4-yl)-2-(triphenylmethoxyimino)acetic acidwith tributyl-amine;1,4-diaza-bicyclo[2.2.2]octane in dichloromethane at 20 - 30; for 0.333333 h;Inert atmosphere;
Stage #2: diethyl phosphorochloridothioate in dichloromethane at 2; for 0.5 h;
Steps:
3
Example 3Preparation of (Z)-(2-aminothiazol-4-yl)triphenylmethyloxyiminoacetic acid diethoxythio- phosphoryl ester30 g (0.070 mol) of (Z)-(2-aminotmazol-4-yl)triphenylmethyloxyiminoacetic acid obtained in Example 2, 0.08 g (1 mmol) of DABCO and 150 mL of CH2C12 were charged into a reactor at 25 +/- 5 °C. The reactor was inerted with N2, and then 19.42 g (0.105 mol) of tributyiamine was added dropwise during 15 min at 25 +/- 5 °C. The reaction mixture was stirred for 5 min and cooled to 2 °C. Then, 19.76 g (0.105 mol) of diethyl chlorothiophosphate was added over a period of 30 min. After the addition was completed, the reaction mixture was stirred until (Z)-(2-aminothiazol-4-yl)triphenylmethyloxyiminoacetate reached <1.0 area% on HPLC (about 4 h).To the above mixture, 150 mL of water was added over a period of 45 min at 2 °C. The mixture was stirred for 15 min and the aqueous phase was removed. 150 mL of ?-hexane was added at 2 °C during 40 min. The suspension was stirred at 2 °C for 1 h, and then the product was filtered and washed with a solution of 135 mL of ?-hexane and 45 mL of dichloromethane in 3 portions. The product was filtered and dried for 15 h at 25 °C and 25 mbar to obtain 36.4 g of the title product as a white product solid (98.38% HPLC purity, 89.0% yield). 1H NMR (400 MHz, DMSO-d6): 5 = 1.26 (dt, J= 7.2 Hz, 6H), 4.21^.27 (m, 4H), 6.71 (s, 1 H), 7.27-7.36 (m, 17H).13C NMR (100 MHz, DMSO-d6) δ = 16.0, 16.1, 66.5, 66.6, 92.4, 110.1 , 128.0, 128.3, 129.0, 140.5, 143.7, 146.0, 157.5, 169.7.
References:
WO2011/29596,2011,A1 Location in patent:Page/Page column 8

64485-82-1
158 suppliers
$9.00/5g
![4-THIAZOLEACETIC ACID, 2-AMINO-ALPHA-[(TRIPHENYLMETHOXY)IMINO]-, ANHYDRIDE WITH O,O-DIETHYL HYDROGEN PHOSPHOROTHIOATE, (Z)-](/CAS/GIF/200814-98-8.gif)
200814-98-8
19 suppliers
inquiry
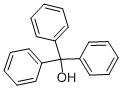
76-84-6
419 suppliers
$6.00/25g
![4-THIAZOLEACETIC ACID, 2-AMINO-ALPHA-[(TRIPHENYLMETHOXY)IMINO]-, ANHYDRIDE WITH O,O-DIETHYL HYDROGEN PHOSPHOROTHIOATE, (Z)-](/CAS/GIF/200814-98-8.gif)
200814-98-8
19 suppliers
inquiry