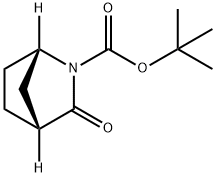
2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)- synthesis
- Product Name:2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)-
- CAS Number:204913-00-8
- Molecular formula:C11H17NO3
- Molecular Weight:211.26

24424-99-5
826 suppliers
$13.50/25G
![(1S,4R)-2-Aza-bicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/130931-83-8.gif)
130931-83-8
169 suppliers
$8.00/100mg
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)-](/CAS/20180601/GIF/204913-00-8.gif)
204913-00-8
15 suppliers
inquiry
Yield:204913-00-8 86%
Reaction Conditions:
Stage #1: (1S,4R)-2-azabicyclo[2,2,1]hept-5-en-3-onewith hydrogen;palladium 10% on activated carbon in methanol; under 2585.81 Torr; for 1 h;
Stage #2: di-tert-butyl dicarbonatewith dmap in dichloromethane at 20;
Steps:
61.A
50 g (0.46 mol) of (1S,4R)-(+)-2-azabicyclo[2.2.1]hept-5-en-3-one in 200 mL of methanol containing 2.5 g of Pd/C (10%) was hydrogenated on a Parr Apparatus under 50 psi of hydrogen for 1 h. The catalyst was removed by filtration through a pad of celite. The filtrates were evaporated and the residue was dried in vacuum. The resulting white solid (50 g) was dissolved in 200 mL of methylene chloride and 110 g (0.50 mol) of di-tert-butyl dicarbonate and 1.0 g of DMAP were added. The reaction mixture was stirred at room temperature overnight and then loaded on a silica gel column, eluted with 10% EtOAc/Hexane. The title compound (83 g, 86%) was obtained as a white solid. 1NMR (400 MHz, CDCl3): 1.40 (d, 1H), 1.51 (s,9H), 1.70-1.95 (m, 5H), 2.84 (m, 1H), 4.50 (m, 1H).
References:
US2007/117797,2007,A1 Location in patent:Page/Page column 33

24424-99-5
826 suppliers
$13.50/25G
![2-Azabicyclo[2.2.1]heptan-3-one, 2-(9-phenyl-9H-fluoren-9-yl)-, (1S,4R)-](/CAS/20210305/GIF/147698-14-4.gif)
147698-14-4
0 suppliers
inquiry
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)-](/CAS/20180601/GIF/204913-00-8.gif)
204913-00-8
15 suppliers
inquiry

24424-99-5
826 suppliers
$13.50/25G
![(1S,4R)-2-AZABICYCLO[2.2.1]HEPTAN-3-ONE](/CAS/GIF/134003-03-5.gif)
134003-03-5
18 suppliers
$733.72/250MG
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)-](/CAS/20180601/GIF/204913-00-8.gif)
204913-00-8
15 suppliers
inquiry
![(6R,7S)-2-BOC-2-AZA-BICYCLO[2.2.1]HEPT-5-EN-3-ONE](/CAS/GIF/151792-53-9.gif)
151792-53-9
46 suppliers
inquiry
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)-](/CAS/20180601/GIF/204913-00-8.gif)
204913-00-8
15 suppliers
inquiry
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
225 suppliers
$10.00/1g
![2-Azabicyclo[2.2.1]heptane-2-carboxylic acid, 3-oxo-, 1,1-dimethylethyl ester, (1S)-](/CAS/20180601/GIF/204913-00-8.gif)
204913-00-8
15 suppliers
inquiry