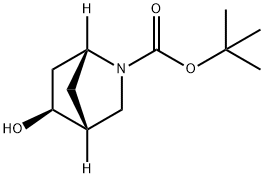
(5S)-tert-butyl 5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate synthesis
- Product Name:(5S)-tert-butyl 5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate
- CAS Number:207405-69-4
- Molecular formula:C11H19NO3
- Molecular Weight:213.27
![(1R,4S)-tert-butyl 2-aza-bicyclo[2.2.1]hept-5-ene-2-carboxylate](/CAS/GIF/702666-73-7.gif)
702666-73-7
1 suppliers
inquiry
![(5S)-tert-butyl 5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate](/CAS/20130318/GIF/CB82599793.gif)
207405-69-4
13 suppliers
inquiry
Yield:207405-69-4 61%
Reaction Conditions:
Stage #1: tert-butyl (1R,4S)-(+)-2-azabicyclo[2.2.1]hept-5-ene-2-carboxylatewith sodium tetrahydroborate in tetrahydrofuran at 23; for 0.5 h;Inert atmosphere;
Stage #2: with dimethyl sulfate in tetrahydrofuran at 35; for 4 h;
Stage #3: with water;dihydrogen peroxide;sodium hydroxide in tetrahydrofuran at 0 - 23; for 1 h;
Steps:
B.B3
(1R,4R,5S)-tert-Butyl 5-hydroxy-2-azabicyclo[2.2.1]heptanes-2-carboxylate (B3) The mixture of (1R,4S)-tert-butyl 2-azabicyclo[2.2.1]hept-5-ene-2-carboxylate (1.50 g, 7.68 mmol) and sodium borohydride (0.24 g, 6.30 mmol) in THF (9.5 mL) was stirred for 0.5 h under nitrogen atmosphere at 23° C. After being stirred for 0.5 h, the mixture was warmed to 35° C. and then dimethylsulfate (0.57 mL, 6.30 mmol) dissolved in THF (2.0 mL) was added dropwise via syringe. The resulting mixture was stirred for 4 h at 35° C., then cooled to 0° C. and quenched by dropwise addition of H2O (5.0 mL). A solution of sodium hydroxide (15.0 mL, 15.0 mmol, 1 M solution of NaOH) was added at 0° C. followed by addition of hydrogen peroxide (0.96 mL, 30 wt. % in H2O). The mixture was warmed to 23° C. and stirred for additional 1 h. The resulting colorless solution was diluted with diethyl ether (75.0 mL) and the organic layer was separated, washed with brine (50.0 mL) and dried over magnesium sulfate. The mixture was concentrated by rotavap and the resulting colorless oil as crude product was purified by column chromatography (SiO2, EtOAc:n-Hex 1:1 (v/v)) to provide the title compound B3 (1.00 g, 4.69 mmol, 61%) as a colorless oil.
References:
US2012/238751,2012,A1 Location in patent:Page/Page column 35
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
222 suppliers
$10.00/1g
![(5S)-tert-butyl 5-hydroxy-2-azabicyclo[2.2.1]heptane-2-carboxylate](/CAS/20130318/GIF/CB82599793.gif)
207405-69-4
13 suppliers
inquiry