
4-(4-FORMYL-PHENOXY)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-(4-FORMYL-PHENOXY)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:207798-38-7
- Molecular formula:C17H23NO4
- Molecular Weight:305.37

123-08-0
905 suppliers
$5.00/10g
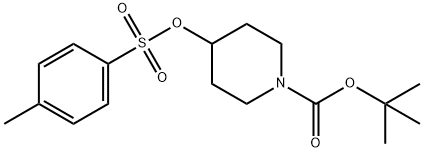
118811-07-7
118 suppliers
$9.00/250mg

207798-38-7
14 suppliers
inquiry
Yield:207798-38-7 100%
Reaction Conditions:
with potassium carbonate in acetonitrile at 80; for 18 h;
Steps:
Step (i): Preparation of t-Butyl 4-(4-formyl phenoxy) piperidine-1-carboxylate
Step (i): Preparation of t-Butyl 4-(4-formyl phenoxy) piperidine-1-carboxylateA solution of 4-Hydroxybenzaldehyde (20.1 grams, 0.164 moles), potassium carbonate (67.9 grams, 0.492 moles) and t-butyl 4-(toluene-4-sulfonyloxy) piperidine-l-carboxylate (70 grams, 0.197 moles) in acetonitrile (1000 mL) was stirred for 18 hours at 80 °C. The progress ofthe reaction was monitored by thin layer chromatography. After completion of reaction, the mass was cooled to room temperature and quenched on to chilled water (1000 mL). The compound was extracted with dichloromethane (3 x 500 mL). The resulting dichloromethane layer was washed with 10 % lye solution (100 mL), water (100 mL) and brine solution (100 mL). The organic phase was dried over sodium sulfate and concentrated under reduced pressure to afford the titlecompound (50.3 grams).Yield: 100 %.‘H - NMR (6 ppm): 1.48 (9H, s), 1.76 - 1.83 (2H, m), 1.96 - 2.04 (2H, m), 3.36 - 3.41 (2H, m), 3.68 - 3.73 (2H, m), 4.45 - 4.60 (IH, m), 6.96 - 7.02 (2H, m), 7.78 - 7.85 (2H, m), 9.89 (IH, s);Mass (m/z): 306.4 (M+H).
References:
WO2014/30170,2014,A1 Location in patent:Page/Page column 5

123-08-0
905 suppliers
$5.00/10g

109384-19-2
470 suppliers
$5.00/1g

207798-38-7
14 suppliers
inquiry

123-08-0
905 suppliers
$5.00/10g
141699-59-4
236 suppliers
$6.00/1g

207798-38-7
14 suppliers
inquiry

10465-78-8
172 suppliers
$10.00/250mg

123-08-0
905 suppliers
$5.00/10g

109384-19-2
470 suppliers
$5.00/1g

207798-38-7
14 suppliers
inquiry
4143-61-7
4 suppliers
inquiry

123-08-0
905 suppliers
$5.00/10g

109384-19-2
470 suppliers
$5.00/1g

207798-38-7
14 suppliers
inquiry