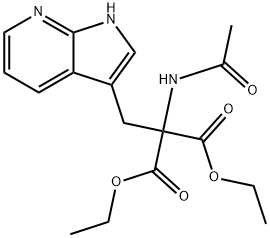
Ethyl α-Acetamido-α-carbethoxy-β-(7-aza-3-indolyl)propionate synthesis
- Product Name:Ethyl α-Acetamido-α-carbethoxy-β-(7-aza-3-indolyl)propionate
- CAS Number:211179-97-4
- Molecular formula:C17H21N3O5
- Molecular Weight:347.37
![1-(2,9-diazabicyclo[4.3.0]nona-2,4,7,10-tetraen-7-yl)-N,N-dimethyl-methanamine](/CAS/GIF/5654-92-2.gif)
5654-92-2
73 suppliers
$30.00/250mg
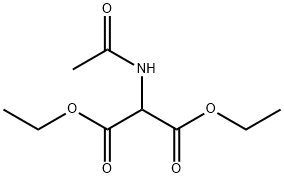
1068-90-2
550 suppliers
$5.00/25g

211179-97-4
19 suppliers
inquiry
Yield:211179-97-4 50.4%
Reaction Conditions:
with sodium hydroxide in 5,5-dimethyl-1,3-cyclohexadiene; for 15 h;Reflux;Inert atmosphere;
Steps:
1
Step 1: A mixture of 7-azagramine (3.5 g, 19.97 mmol), diethyl acetamidomalonate (4.34 g, 19.97 mmol) and xylenes (35 ml) was treated with powder sodium hydroxide (0.080 g, 1.997 mmol) and stirred at reflux for 15 h under nitrogen. The hot solution was filtered to give a yellow filtrate. A yellow solid precipitated from the filtrate when cooled to room temperature. The solid mass was suspended in benzene (40 mL) and filtered. Thecollected solid was washed with cyclohexane (2 x 100 mL) to give diethyl 2-((1H- pyrrolo[2,3-b]pyridin-3-yl)methyl)-2-acetamidomalonate (3.5 g, 50.4 %) as a white solid. Analysis LCMS Condition A: Retention time = 0.79 mm; ESI-MS(+) m/z 348.3 (M+H).
References:
WO2016/39749,2016,A1 Location in patent:Page/Page column 190