2-((5-Bromopyridin-2-yl)oxy)-N,N-dimethylethanamine synthesis
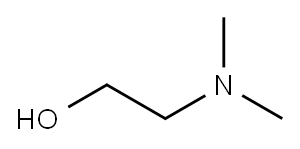
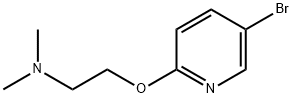
- Product Name:2-((5-Bromopyridin-2-yl)oxy)-N,N-dimethylethanamine
- CAS Number:212961-35-8
- Molecular formula:C9H13BrN2O
- Molecular Weight:245.12
Yield:212961-35-8 94%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 2 h;
Steps:
The synthesis of intermediate E
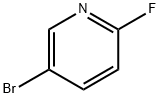
A mixture of 5-bromo-2-fluoropyridine (0.31 mL, 3 mmol) and 2-(dimethylamino)ethanol (0.25 mL, 2.5 mmol) in DMF (10 mL) in a 100 mL was chilled to 0 °C. The mixture was then treated with NaH (120 mg, 5.00 mmol) and allowed to warm to room temperature and stirred for 2 h or until complete by LC/MS. The mixture was then treated with water to quench, and extracted with ethyl acetate. The organic layer was collected, dried, filtered, and concentrated. The residue was purified by SiO2 chromatography (DCM-MeOH 0-20%) to give desired intermediate E (575 mg, 94%).
References:
Jiang, Jian-kang;Huang, Xiuli;Shamim, Khalida;Patel, Paresma R.;Lee, Arthur;Wang, Amy Q.;Nguyen, Kimloan;Tawa, Gregory;Cuny, Gregory D.;Yu, Paul B.;Zheng, Wei;Xu, Xin;Sanderson, Philip;Huang, Wenwei [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 20,p. 3356 - 3362] Location in patent:supporting information
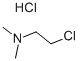
4584-46-7
5 suppliers
$19.73/100g
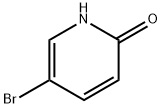
13466-38-1
388 suppliers
$6.00/5g

212961-35-8
18 suppliers
inquiry