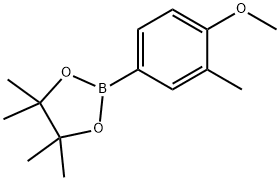
2-(4-methoxy-3-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(4-methoxy-3-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:214360-63-1
- Molecular formula:C14H21BO3
- Molecular Weight:248.13
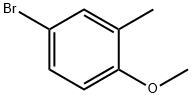
14804-31-0
152 suppliers
$7.00/5g

73183-34-3
568 suppliers
$6.00/5g

214360-63-1
25 suppliers
inquiry
Yield:214360-63-1 54%
Reaction Conditions:
Stage #1: 2-methyl-4-bromoanisole;bis(pinacol)diboranewith potassium acetate in dimethyl sulfoxide; for 0.166667 h;
Stage #2: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in dimethyl sulfoxide at 80; for 24 h;
Steps:
1.a Example 1 (Entry 1013); 4'-[2-(11-Ethyl-6,11-dihydro-5-methyl-6-oxo-5H-dipyrido[3,2-b:2',3'-e][1,4]diazepin-8-yl)ethoxy]-3'-methyl-[1,1'-biphenyl]-3-carboxylic Acid; a) 2-(4-Methoxy-3-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxoborolane
A mixture of bis(pinacolato)diborane (10.7 g, 42.1 mmol), 4-bromo-1-methoxy-2-methylbenzene (7.70 g, 38.3 mmol) and KOAc (10.9 g, 114 mmol) in DMSO (200 mL) was degassed with argon for 10 min. PdCl2dppf (1:1 complex with CH2Cl2, 2.50 g, 3.06 mmol) was next added and the reaction mixture was heated to 80° C. for 24 h. The reaction mixture was diluted with water and extracted with C6H6 (3×). The combined organic layers were washed with brine, dried (MgSO4), filtered and concentrated under reduced pressure. The residue was purified by flash chromatography (Hexane:EtOAc, 95:5) to yield the title compound (5.1 g, 54% yield) as a colorless oil.
References:
US2004/6071,2004,A1 Location in patent:Page/Page column 13

75581-11-2
62 suppliers
$9.00/1g
25015-63-8
220 suppliers
$22.39/5G

214360-63-1
25 suppliers
inquiry
578-58-5
259 suppliers
$15.00/5G

73183-34-3
568 suppliers
$6.00/5g

214360-63-1
25 suppliers
inquiry

1417036-28-2
27 suppliers
$102.00/5g
3260-85-3
91 suppliers
$19.00/1g

214360-63-1
25 suppliers
inquiry