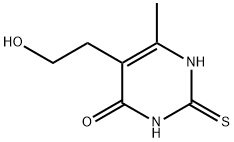
2,3-dihydro-5-(2-hydroxyethyl)-6-methyl-2-thioxo-1H-pyrimidin-4-one synthesis
- Product Name:2,3-dihydro-5-(2-hydroxyethyl)-6-methyl-2-thioxo-1H-pyrimidin-4-one
- CAS Number:21585-16-0
- Molecular formula:C7H10N2O2S
- Molecular Weight:186.23
517-23-7
447 suppliers
$5.00/25g

17356-08-0
3 suppliers
inquiry

21585-16-0
19 suppliers
$45.00/100mg
Yield:21585-16-0 56%
Reaction Conditions:
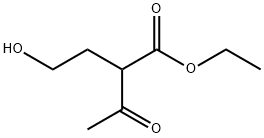
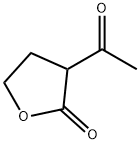
Stage #1: 3-acetyl-2-oxo-4,5-dihydrofuran;thioureawith ethanol;sodium for 16 h;Heating / reflux;
Stage #2: with hydrogenchloride in water;
Steps:
2.a
To a solution of sodium (55.6 g, 2.4 mol) in ethanol (1000 ml) was added slowly 2-acetylbutyrolactone 155 g, 1.2 mol) after which thiourea (128 g, 1.65 mol) was added in small portions. The mixture was heated under reflux for 16 h, then concentrated, water (800 ml) was added and acidified slowly with concentrated hydrochloric acid (200 ml). The precipitate was collected and washed with water, isopropyl alcohol and petroleum ether. Yield 125 g (56%) of a white solid.
References:
US2006/122206,2006,A1 Location in patent:Page/Page column 4