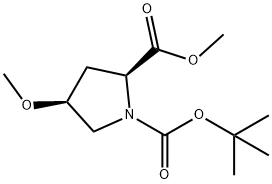
(2S,4S)-1-tert-butyl 2-methyl 4-methoxypyrrolidine-1,2-dicarboxylate synthesis
- Product Name:(2S,4S)-1-tert-butyl 2-methyl 4-methoxypyrrolidine-1,2-dicarboxylate
- CAS Number:215918-38-0
- Molecular formula:C12H21NO5
- Molecular Weight:259.3
87691-27-8
222 suppliers
$6.00/250mg

74-88-4
340 suppliers
$15.00/10g

215918-38-0
24 suppliers
$75.00/250MG
Yield:215918-38-0 550 mg
Reaction Conditions:
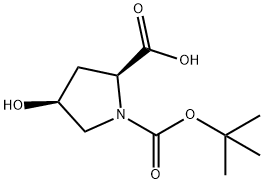
Stage #1: N-tert-butoxycarbonyl-L-cis-4-hydroxyprolinewith sodium hydride in N,N-dimethyl-formamide; for 0.5 h;Cooling with ice;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20;
Steps:
A
To an ice cooled mixture of Sodium hydride (228 mg, 5.71 mmol)(prewashed with hexanes) and DMF (10 mL) was added (2S,4S)-l-(tert- butoxycarbonyl)-4-hydroxypyrrolidine-2-carboxylic acid (600 mg, 2.59 mmol) as a solid in one portion. The formed slurry was stirred at this temperature for 30 min before addition of iodomethane (0.485 mL, 7.78 mmol) dropwise. The final reaction mixture was stirred at rt overweekend. Poured into water (100 mL), extracted with EtOAc (20 mL, X2). The combined organic layer was washed with water and brine, dried over MgS04, filtered, evaporated in vacuo. The residue was purified by FCC (0% to 50% EtOAc-Hexanes) to afford Cap W-19 Step A (550 mg) as a colorless oil. NMR (500MHz, CD3OD) δ 4.38 (td, J=8.6, 3.2 Hz, 1H), 3.99 (dt, J=5.1, 2.4 Hz, 1H), 3.81 - 3.68 (m, 3H), 3.64 - 3.53 (m, 1H), 3.50 - 3.40 (m, 1H), 2.45 - 2.21 (m, 2H), 1.55 - 1.40 (m, 9H)
References:
WO2015/5901,2015,A1 Location in patent:Page/Page column 607

24424-99-5
821 suppliers
$13.50/25G
75176-19-1
0 suppliers
inquiry

215918-38-0
24 suppliers
$75.00/250MG
13504-86-4
83 suppliers
$18.00/100mg

215918-38-0
24 suppliers
$75.00/250MG

121147-94-2
14 suppliers
inquiry

215918-38-0
24 suppliers
$75.00/250MG
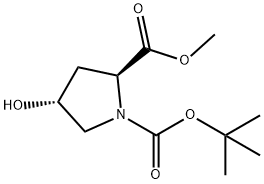
74844-91-0
405 suppliers
$5.00/5g

215918-38-0
24 suppliers
$75.00/250MG