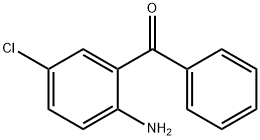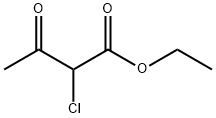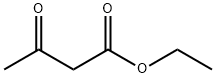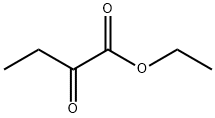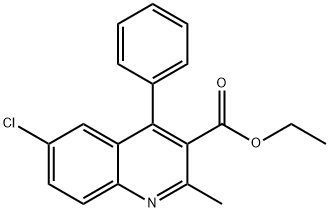
6-Chloro-3-ethoxycarbonyl-2-methyl-4-phenylquinoline synthesis
- Product Name:6-Chloro-3-ethoxycarbonyl-2-methyl-4-phenylquinoline
- CAS Number:22609-01-4
- Molecular formula:C19H16ClNO2
- Molecular Weight:325.79
Yield:22609-01-4 89%
Reaction Conditions:
with sodium salt of sulfated chitosan in ethanol at 50; for 0.333333 h;Friedlaender Quinoline Synthesis;
Steps:
General procedure
General procedure: A mixture of 2-aminoarylketone (1.0 mmol), α-methyleneketone (1.0 mmol) and chitosan-SO3H (obtained from Aldrich) (100 mg) in ethanol (5 mL) was stirred at 50 °C for the specified time (see Table 1). After completion, as monitored by TLC, the catalyst was separated by filtration and the residue was washed with ethanol (5 mL). The combined organic layers were concentrated under reduced pressure and the crude product was purified by silica gel column chromatography using ethyl acetate-n-hexane (1:9) as eluent to afford the pure quinoline derivative. The products 3a, 3b, 3c, 3d, 3e, 3f, 3g, 3h, 3i, 3k, 3j, 3m are known in literature.30 Spectral data for the new products:
References:
Reddy, B.V. Subba;Venkateswarlu;Reddy, G. Niranjan;Reddy, Y.V. Rami [Tetrahedron Letters,2013,vol. 54,# 43,p. 5767 - 5770]