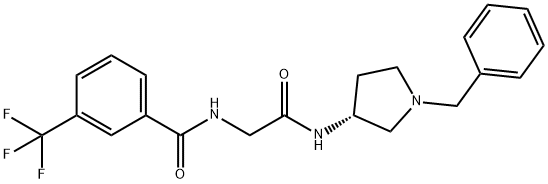
Benzamide, N-[2-oxo-2-[[(3R)-1-(phenylmethyl)-3-pyrrolidinyl]amino]ethyl]-3-(trifluoromethyl)- synthesis
- Product Name:Benzamide, N-[2-oxo-2-[[(3R)-1-(phenylmethyl)-3-pyrrolidinyl]amino]ethyl]-3-(trifluoromethyl)-
- CAS Number:226228-88-2
- Molecular formula:C21H22F3N3O2
- Molecular Weight:405.41
![2-[3-(Trifluoromethyl)benzoyl]aminoacetic acid](/CAS/GIF/17794-48-8.gif)
17794-48-8
118 suppliers
$20.00/100mg
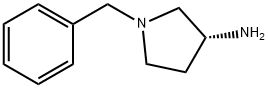
114715-39-8
231 suppliers
$18.00/250mg
![Benzamide, N-[2-oxo-2-[[(3R)-1-(phenylmethyl)-3-pyrrolidinyl]amino]ethyl]-3-(trifluoromethyl)-](/CAS/20180703/GIF/226228-88-2.gif)
226228-88-2
7 suppliers
inquiry
Yield:226228-88-2 69%
Reaction Conditions:
Stage #1: m-trifluoromethylhippuric acidwith 4-methyl-morpholine;isobutyl chloroformate in tetrahydrofuran at -10;Inert atmosphere;
Stage #2: (R)-1-benzyl-3-aminopyrrolidine in tetrahydrofuran at -10; for 1 h;Inert atmosphere;
Steps:
2
Manufacturing Example 2 N-[(1-benzylpyrrolidine-(3R)-yl-carbamoyl)-methyl]-3-trifluoromethylbenzamide 10.74 g (43.4 mmol) (3-trifluoromethylbenzoylamino)-acetic acid and 6.58 g (65.10 mmol, 1.5 eq.) N-methylmorpholine described at manufacturing example 1 dissolved into 80 ml tetrahydrofuran under argon gas. After cooling at -10° C., 7.11 g (52.08 mmol, 1.2 eq.) of isobutylchloroformate was diluted with 10 ml tetrahydrofuran and was added dropwise slowly into reaction solution. It was stirred at the same temperature and 8.03 g (45.57 mmol, 1.1 eq.) (3R)-(-)-1-benzyl-3-aminopyrrolidine was diluted with 10 ml tetrahydrofuran and was added dropwise slowly. After mixing at -10° C. for one hour, 100 ml purified water was added. It was three times continuously extracted with 100 ml ethyl acetate and organic layer was recovered. It was dried with anhydrous magnesium sulfate, decompressed and concentrated. After residues are solidified with t-butylmethylether and filtered, 12.18 g (69%) target compound as white solid was yielded. 1H NMR (400 MHz, DMSO-d6) 1.62-1.66 (1H, m), 2.26-2.36 (2H, m), 2.54-2.59 (1H, m), 2.63-2.66 (1H, m), 2.89-2.93 (1H, m), 3.62 (2H, d), 4.10 (2H, d), 4.46-4.90 (1H, m), 6.45 (1H, br s), 7.15 (1H, br s), 7.29-7.34 (5H, m), 7.59 (1H, t), 7.78 (1H, d), 8.00 (1H, d), 8.11 (1H, s)
References:
US2012/190689,2012,A1 Location in patent:Page/Page column 12
![2-[3-(Trifluoromethyl)benzoyl]aminoacetic acid](/CAS/GIF/17794-48-8.gif)
17794-48-8
118 suppliers
$20.00/100mg
![Benzamide, N-[2-oxo-2-[[(3R)-1-(phenylmethyl)-3-pyrrolidinyl]amino]ethyl]-3-(trifluoromethyl)-](/CAS/20180703/GIF/226228-88-2.gif)
226228-88-2
7 suppliers
inquiry
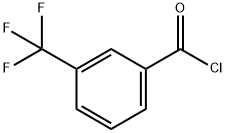
2251-65-2
212 suppliers
$10.00/1g
![Benzamide, N-[2-oxo-2-[[(3R)-1-(phenylmethyl)-3-pyrrolidinyl]amino]ethyl]-3-(trifluoromethyl)-](/CAS/20180703/GIF/226228-88-2.gif)
226228-88-2
7 suppliers
inquiry