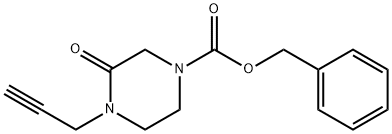
Benzyl 3-oxo-4-(prop-2-ynyl)-piperazine-1-carboxylate synthesis
- Product Name:Benzyl 3-oxo-4-(prop-2-ynyl)-piperazine-1-carboxylate
- CAS Number:234108-42-0
- Molecular formula:C15H16N2O3
- Molecular Weight:272.3
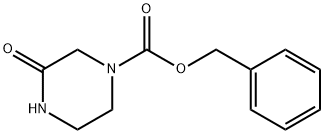
78818-15-2
149 suppliers
$24.73/1gm:

106-96-7
332 suppliers
$31.00/25g

234108-42-0
9 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydride in tetrahydrofuran;DMF (N,N-dimethyl-formamide) at 0 - 20;
Steps:
69.C C. 3-Oxo-4-prop-2-ynylpiperazine-1-carboxylic acid benzyl ester
C. 3-Oxo-4-prop-2-ynylpiperazine-1-carboxylic acid benzyl ester. sodium hydride (0.82 g, 23.0 mmol, 60% mineral oil dispersion) is added to a solution of 4-benzyloxycarbonylpiperazin-2-one (5.13 g, 21.9 mmol) in THF/DMF (75 ML, 3/1 v/v) at 0° C. The mixture is stirred for 5 minutes, then propargyl bromide (3.7 ML, 41.5 mmol) is added dropwise.The resulting solution is stirred for 1 hour then brought to room temperature and stirred for 2 hours.The reaction is quenched with saturated ammonium chloride solution then diluted with ethyl acetate and washed with water (4*) and brine.The organic layer is dried over MgSO4, filtered and concentrated to dryness.The residue is purified by column chromatography eluding with 5% MeOH/CH2Cl2 to give the product (5.96 g, 21.9 mmol) as a white solid. 1H NMR (CDCl3, 300 MHz) δ7.3 (m, 5H), 5.12 (s, 2H), 4.25 (s,2H), 4.16 (s, 2H), 3.75 (m, 2H), 3.47 (m, 2H), 2.22 (s, 1H).
References:
US2004/102450,2004,A1 Location in patent:Page/Page column 86