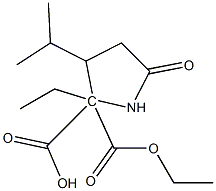
Diethyl=3-isopropyl-5-oxo-2,2-pyrrolidinedicarboxylate synthesis
- Product Name:Diethyl=3-isopropyl-5-oxo-2,2-pyrrolidinedicarboxylate
- CAS Number:2445-91-2
- Molecular formula:C13H21NO5
- Molecular Weight:271.31
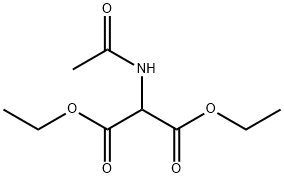
1068-90-2
541 suppliers
$5.00/25g

2445-91-2
1 suppliers
inquiry
Yield:2445-91-2 3.5 g (45.9%)
Reaction Conditions:
with sodium in ethanol;
Steps:
17.B 17-B.
17-B. 5,5-Diethoxycarbonyl-4-(1-methyl)ethyl-2-pyrrolidinone To a stirred solution of sodium (100 mg) in absolute dry ethanol (40 ml) was added diethyl acetamidomalonate (4.07 g, 18.75 mmol) at room temperature under an atmosphere of nitrogen. After 10 minutes, compound 17-A (4 g, 28.13 mmol) was slowly added. After the addition was complete, the mixture was heated under reflux for 20 hours. The mixture was then allowed to come to room temperature and neutralized with acetic acid. The ethanol was evaporated in vacuo. The residual slurry was diluted with brine and extracted with ethyl acetate (120 ml). The ethyl acetate extract was washed with water, dried over anhydrous magnesium sulfate and evaporated in vacuo to give a solid. This solid was recrystallized from ethyl ether-hexane to give 3.5 g (45.9%) of thin layer chromatography-homogeneous compound 17-B as a colorless solid, melting point 95°-96° C., with consistent 1 H-NMR and 13 C-NMR spectra. See, for example, A. B. Mauger, J. Org. Chem.; 46, 1032 (1981) and W. Hartwig and L. Born, J. Org. Chem., 52, 4352 (1987).
References:
US5049577,1991,A