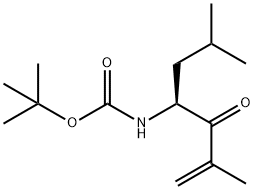
CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI) synthesis
- Product Name:CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI)
- CAS Number:247068-81-1
- Molecular formula:C14H25NO3
- Molecular Weight:255.35
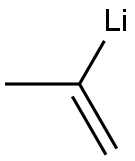
6386-71-6
0 suppliers
inquiry

87694-50-6
64 suppliers
$13.50/1G
![CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI)](/CAS/20150408/GIF/247068-81-1.gif)
247068-81-1
90 suppliers
$19.00/250mg
Yield:247068-81-1 92%
Reaction Conditions:
in diethyl ether at -78; for 2.5 h;
Steps:
The second (right hand) molecular fragment can be made in parallel or in sequence. This procedure involves alkenylating a Weinreb amide of an amino terminal protected amino acid. For example, an amino terminal protected amino acid can be reacted with a reagent (for example, MeNHOMeHCl, EDCl, NMM, HoBt, DMF, 0, 12 h, 80%) to make a Weinreb amide of the carboxyl terminus of the amino acid. Alkenylation is carried out by exposing the Weinreb amide to an alkenylating agent (for example, propen-2-yl lithium) to give an ?','-unsaturated ketone. For example, for an epoxomicin synthesis, Boc-Leu Weinreb amide can be reacted with propen-2-yl lithium to give the corresponding ?','-unsaturated ketone. Propen-2-yl lithium can be generated by reaction of 2-bromopropene with t-butyl lithium in a non-protic solvent (for example, diethyl ether) at low temperature (for example -78 C.), for at least 30 minutes (for example 2.5 hours) to give the ?','-unsaturated ketone in excellent yield (for example, 92%).
References:
US6831099,2004,B1 Location in patent:Page column 13-14
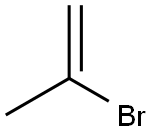
557-93-7
211 suppliers
$45.10/5g

87694-50-6
64 suppliers
$13.50/1G
![CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI)](/CAS/20150408/GIF/247068-81-1.gif)
247068-81-1
90 suppliers
$19.00/250mg
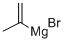
13291-18-4
159 suppliers
$133.00/25ml

87694-50-6
64 suppliers
$13.50/1G
![CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI)](/CAS/20150408/GIF/247068-81-1.gif)
247068-81-1
90 suppliers
$19.00/250mg

87694-50-6
64 suppliers
$13.50/1G

106-95-6
417 suppliers
$10.00/5g
![CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI)](/CAS/20150408/GIF/247068-81-1.gif)
247068-81-1
90 suppliers
$19.00/250mg

557-93-7
211 suppliers
$45.10/5g

173899-64-4
2 suppliers
inquiry
![CarbaMic acid, [(1S)-3-Methyl-1-(2-Methylpropyl)-2-oxo-3-butenyl]-, 1,1-diMethylethyl ester (9CI)](/CAS/20150408/GIF/247068-81-1.gif)
247068-81-1
90 suppliers
$19.00/250mg