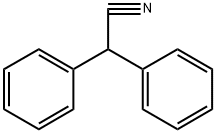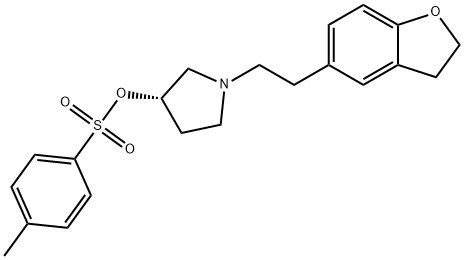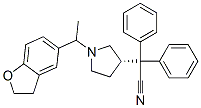
{(3S)-1-[1-(2,3-DIHYDRO-1-BENZOFURAN-5-YL)ETHYL]PYRROLIDIN-3-YL}DIPHENYLACETONITRILE synthesis
- Product Name:{(3S)-1-[1-(2,3-DIHYDRO-1-BENZOFURAN-5-YL)ETHYL]PYRROLIDIN-3-YL}DIPHENYLACETONITRILE
- CAS Number:252317-48-9
- Molecular formula:C28H28N2O
- Molecular Weight:408.53
Yield:-
Reaction Conditions:
with lithium amide in N,N-dimethyl-formamide;toluene at 110;
Steps:
4
EXAMPLE 4 Obtaining 3-(S)-(-)-(1-carbonitrile-1,1-diphenylmethyl)-1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]pyrrolidine hydrobromide (S-Ia) [Show Image] 6.13 g (0.0317 moles) of diphenylacetonitrile were slowly added to a solution consisting of 10.3 g (0.0264 moles) of 3-(S)-1-[2-(2,3-dihydrobenzofuran-5-yl)ethyl]-3-p-toluenesulfonylpyrrolidine (S-IIa), 1.22 g (0.0529 moles) of NH2Li, 20.5 ml of DMF and 102.5 ml of toluene heated at 110°C. The reaction mixture was maintained under stirring at said temperature (toluene reflux) for about 5 hours. Once the reaction was finished, the mixture was cooled at 15°C and 100 ml of water were added. After the separation of the phases, the organic phase was washed consecutively with 1N HCl aqueous solution (2x50 ml) and 20% NaOH aqueous solution (2x50 ml). The organic phase was concentrated to a residue at reduced pressure and was redissolved in 30 ml of acetone. Approximately 0.75 ml (0.013 moles) of 33% HBr in acetic acid were added little by little to this solution stirred at room temperature until reaching a pH of 3-4. The obtained suspension was maintained under stirring for 1 hour at room temperature and another hour at 0-5°C. The resulting solid was isolated by filtration to give 9.06 g (70% yield) of a white solid having the following spectroscopic properties: [α]25365= 64° (c 0.1, MeOH) free base[α]25365= -21° (c 0.1, MeOH) hydrobromide 1H-NMR (400 MHz, CDCl3): 1.81 (1H, m), 2.04 (1H, m), 2.44-2.57 (2H, m), 2.68 (4H, m), 2.75 (1H, dd, J1=8Hz, J2=16Hz), 2.99 (1H, dt, J1=8Hz, J2=12Hz), 3.12 (1H, t, J=8Hz), 3.42 (1H, m), 4.49 (1H, t, J=8Hz), 6.66 (1H, d, J=8Hz), 6.88 (1H, d, J=8Hz), 7.00 (1H, s), 7.24-7.45 (10H, m) ppm. 13C-NMR (100 MHz, CDCl3): 28.65 (CH2), 29.88 (CH2), 34.89(CH2), 44.85 (CH), 54.08 (CH2), 56.93 (C), 57.97 (CH2), 58.41 (CH2), 71.23 (C).109.06 (CH), 121.65 (C), 125.18 (CH), 127.29 (C), 126.56 (CH), 127.06 (CH), 127.90 (CH), 129.02 (CH), 132.22 (C), 139.92 (C), 140.11 (C), 158.51 (C) ppm. LRMS (electrospray, positive ion): m/z [M++H+] 409.4
References:
EP2236509,2010,A1 Location in patent:Page/Page column 16-17