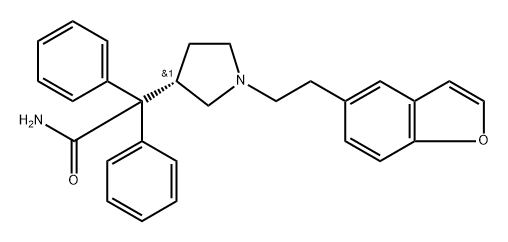
Darifenacin Oxidized IMpurity synthesis
- Product Name:Darifenacin Oxidized IMpurity
- CAS Number:133033-99-5
- Molecular formula:C28H28N2O2
- Molecular Weight:424.54

133033-99-5
15 suppliers
inquiry
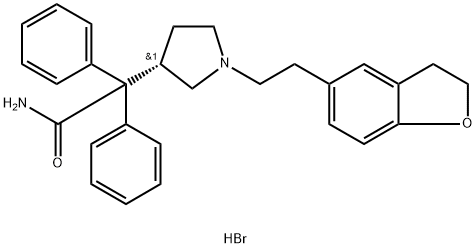
133099-07-7
242 suppliers
$5.00/25mg
Yield:133099-07-7 85%
Reaction Conditions:
Stage #1: (S)-2-{1-[2-(benzofuran-5-yl)ethyl]-3-pyrrolidinyl}-2,2-diphenylacetamidewith hydrogen;acetic acid;palladium 10% on activated carbon at 45 - 50; for 6 - 7 h;
Stage #2: with hydrogen bromide in water;butan-1-ol;Product distribution / selectivity;
Steps:
22
Example 22; Synthesis of (SyZ-n-^-^.S-dihydrobenzofuran-S-vnethyli-S-pyrrolidinvU- 2,2-diphenylacetamide fPRF.HBf); [00174] Pd/C 10% (0.8g) was added to a solution of S)-2-{l-[2-(benzofuran-5- yl)ethyl]-3-pyrrolidinyl}-2,2-diphenylacetaraide (8g) in acetic acid (160 ml). The mixture was hydrogenated at 45-500C and at atmospheric pressure for about 6-7 hrs. Catalyst was filtered off and solution was concentrated under vacuum obtaining an oily residue, n- Butanol (40 ml) was added and stirred to obtain a solution. 48% HBr (3,5 g) was added and a mixture n-Butanol/water was eliminated by distillation to reduce water content to less then 1%. Darifenacin .HBr crystallizes and after 2 hrs at 15-200C was filtered and washed with n-Butanol ( 3x5ml). After drying at 55-600C under vacuum 8.1 g of Darifenacin hydrobromide were obtained. (Yield 85%).
References:
WO2007/76157,2007,A2 Location in patent:Page/Page column 49

133033-99-5
15 suppliers
inquiry
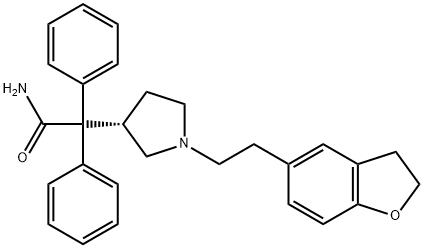
133099-04-4
122 suppliers
$39.00/10mg