
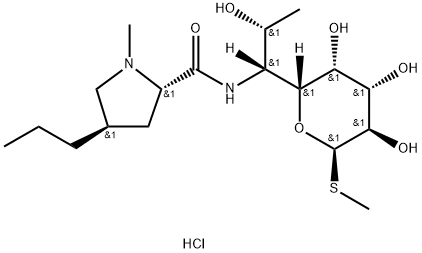
Methyl 6,8-Dideoxy-6-[[[(2S,4R)-1-Methyl-4-propyl-2-pyrrolidinyl]carbonyl]aMino]-1-thio-2,3,4-tris-O-(triMethylsilyl)-D-erythro-α-D-galacto-octopyranoside synthesis
- Product Name:Methyl 6,8-Dideoxy-6-[[[(2S,4R)-1-Methyl-4-propyl-2-pyrrolidinyl]carbonyl]aMino]-1-thio-2,3,4-tris-O-(triMethylsilyl)-D-erythro-α-D-galacto-octopyranoside
- CAS Number:25420-97-7
- Molecular formula:C27H58N2O6SSi3
- Molecular Weight:623.08
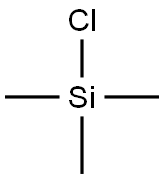
75-77-4
568 suppliers
$5.04/10
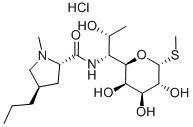
154-21-2
197 suppliers
$115.00/5mg
![Methyl 6,8-Dideoxy-6-[[[(2S,4R)-1-Methyl-4-propyl-2-pyrrolidinyl]carbonyl]aMino]-1-thio-2,3,4-tris-O-(triMethylsilyl)-D-erythro-α-D-galacto-octopyranoside](/CAS/GIF/25420-97-7.gif)
25420-97-7
10 suppliers
$496.00/50mg
Yield:25420-97-7 91%
Reaction Conditions:
Stage #1: chloro-trimethyl-silane;lincomycinwith pyridine;1,1,1,3,3,3-hexamethyl-disilazane at 20; for 2 h;Ice cooling;
Stage #2: with water;acetic acid in methanol at 20; for 16 h;
Stage #3: with sodium hydrogencarbonate in methanol;water;
Steps:
S1.i
Step (i) of Example S1: 2,3,4-Tris-O-trimethylsilyl linc omycin Trimethylsilyl chloride (90 ml, 71 mmol) and 65 ml (60 mmol) of hexamethyldisilazane were added to a solution of 50 g (122 mmol) of lincomycin in pyridine (200 ml) under ice cooling, and the mixture was stirred at room temperature for 2 hr. The solvent was removed by distillation, and the residue was diluted with hexane, followed by washing twice with water. The solvent was removed by distillation. An 80% aqueous acetic acid solution (22.5 ml) was added to a solution of the residue in methanol (150 ml), and the mixture was stirred at room temperature for 16 hr. A saturated aqueous sodium hydrogencarbonate solution (30 ml) was added to the reaction solution. The solvent was removed by distillation, and the residue was then diluted with hexane, followed by washing twice with water. The solvent was removed by distillation to give 69.5 g (yield 91%) of the title compound. 1H-NMR (400 MHz, CDCl3) δ: 0.10-0.20 (27H, m), 0.84-0.92 (3H, m), 1.13 (3H, d, J = 6.3 Hz), 1.21-1.35 (4H, m), 1.75-2.07 (4H, m), 2.08 (3H, s), 3.38 (3H, s), 2.96-3.03 (1H, m), 3.05-3.20 (2H, m), 3.58 (1H, dd, J = 2.2, 9.5 Hz), 3.79 (1H, d, J = 2.2 Hz), 4.00 (1H, d, J = 9.5 Hz), 4.10 (1H, dq, J = 4.9, 6.3 Hz), 4.14 (1H, dd, J = 5.6, 9.5 Hz), 4.33 (1H, ddd, J = 4.9, 9.5, 10.0 Hz), 5.20 (1H, d, J = 5.6 Hz), 7.43 (1H, d, J = 10.0 Hz).
References:
EP2151447,2010,A1 Location in patent:Page/Page column 47

75-77-4
568 suppliers
$5.04/10
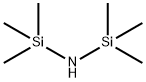
999-97-3
640 suppliers
$9.00/5G

154-21-2
197 suppliers
$115.00/5mg
![Methyl 6,8-Dideoxy-6-[[[(2S,4R)-1-Methyl-4-propyl-2-pyrrolidinyl]carbonyl]aMino]-1-thio-2,3,4-tris-O-(triMethylsilyl)-D-erythro-α-D-galacto-octopyranoside](/CAS/GIF/25420-97-7.gif)
25420-97-7
10 suppliers
$496.00/50mg
859-18-7
507 suppliers
$6.00/100mg
![Methyl 6,8-Dideoxy-6-[[[(2S,4R)-1-Methyl-4-propyl-2-pyrrolidinyl]carbonyl]aMino]-1-thio-2,3,4-tris-O-(triMethylsilyl)-D-erythro-α-D-galacto-octopyranoside](/CAS/GIF/25420-97-7.gif)
25420-97-7
10 suppliers
$496.00/50mg

154-21-2
197 suppliers
$115.00/5mg
![Methyl 6,8-Dideoxy-6-[[[(2S,4R)-1-Methyl-4-propyl-2-pyrrolidinyl]carbonyl]aMino]-1-thio-2,3,4-tris-O-(triMethylsilyl)-D-erythro-α-D-galacto-octopyranoside](/CAS/GIF/25420-97-7.gif)
25420-97-7
10 suppliers
$496.00/50mg